Abstract
The current study describes the phytochemical profile, antimicrobial, mutagenic, and antimutagenic activity of Perovskia atriplicifolia. Benth. essential oil, collected in Pakistan. The sample of essential oil was obtained from aerial parts of the plant by hydrodistillation and analyzed by gas chromatography–mass spectrometry. From the 18 compounds identified, the major compounds were camphor (28.91%), limonene (16.72%), α.-globulol (10.21%), trans.-caryophyllene (9.30%), and α.-humulene (9.25%). Antimicrobial activity of the oil was evaluated using agar diffusion method and agar dilution method. The antimicrobial test results showed that the oil had a significant potential antimicrobial activity against 10 bacteria and 5 fungal strains. Furthermore, the mutagenic and antimutagenic activity of the oil was investigated through the Salmonella./microsome test system, with and without S9 metabolic fraction in Salmonella typhimurium. TA98 and TA100. None of the tested concentrations of oil was found mutagenic. However, all tested concentrations did show an increase in antimutagenic activity with or without S9 fraction against 2-aminofluorene and daunomicina, but not sodium azide. Results presented here suggest that the essential oil of P. atriplicifolia. possesses antimicrobial properties and is therefore a potential source of antimicrobial ingredients for the food and pharmaceutical industry. In addition, that it also has antimutagenic activity raises the importance of this essential oil in this area.
Introduction
Most plants produce antimicrobial secondary metabolites, either as part of their normal pattern of growth and development or in response to pathogen attack or stress. A novel way to reduce the proliferation of microorganisms is by the use of essential oils. The oils are natural products extracted from plant materials, and because of their antibacterial, antifungal, antioxidant, and antimutagenic properties, they can be used as natural additives in many foods (Burt, Citation2004; Ipek et al., Citation2005; Sacchetti et al., Citation2005; Skocibusic et al., Citation2006). Essential oils have proved to be inhibitory against a wide range of food-spoiling microbes, dependent upon their concentration, method of testing, and active constituents (Skocibusic et al., Citation2006).
Perovskia atriplicifolia. Benth. (Lamiaceae) grows wild in the rocky places of Afghanistan and Pakistan. It is used as a cooling medicine in the treatment of fevers. The small lavender flowers have a sweet flavor and may be eaten in salads or used as a garnish. This species is also suitable for use in forming a decorative hedge of moderate height (Pourmortazavi, Citation2003). It is a favorite aromatic shrub for honey bees, with the honey made by bees fed on this shrub being colorless and having a typical flavor and aroma. In a previous work; the oil of P. atriplicifolia. was found to be rich in β.-thujone (12.1%) and 1,8-cineole (10.5%) (Sefidkon et al., Citation1997). The oil of this plant (cultivated in experimental plots at the Botanical Gardens of the University of Turin) was found to contain mainly camphor (14.9%) (Mucciarelli et al., Citation1993). Rao (Citation1926) identified eight components in the oil of this plant. The essential oil of P. atriplicifolia. cultivated in Iran was identified to contain 1,8-cineole + limonene (40.13%), α.-pinene (9.13%), β.-pinene (6.59%), camphene (6.17%), and camphor (5.36%) (Sefidkon et al., Citation1997). Other work on P. atriplicifolia. has been conducted by Dabiri et al., who found that its oil contained different terpenoid compounds at three stages of growth in Iran (Dabiri et al., Citation2001).
More recently, the essential oil of P. atriplicifolia. grown wild in Pakistan has been described with the result of 19 compounds identified (Jassbi et al., Citation1999). The supercritical fluid extraction (SFE) of the aerial parts of P. atriplicifolia. has also been studied with the major components of SFE extracts being 1,8-cineol limonene, camphor, β.-caryophyllene, δ.-cadinene, α.-pinene, and α.-terpinyl acetate (Pourmortazavi, Citation2003). Furthermore, in their studies, the main constituents of the same oil obtained by steam distillation were found to be 1,8-cineol camphene, and α.-humulene (Pourmortazavi, Citation2003).
Although the phytochemical composition of P. atriplicifolia. essential oil was studied previously, to our knowledge, there are no published reports on antimicrobial, mutagenic, and antimutagenic activity of the P. atriplicifolia. essential oil. Therefore, we are focused on the biological activities of the essential oil with the phytochemical composition by gas chromatography–mass spectrometry (GC-MS) analysis.
Materials and Methods
Plant material
The aerial parts of Perovskia atriplicifolia. Benth. were collected from Tira (Khyber Agency), N-W.F.P, Pakistan, in the month of June during flowering season. The plant was identified by Dr. Habib Ahmad, Technical Director of World Wildlife Fund (WWF), Peshawar, Pakistan. A voucher specimen (no. LI-002-JZC) was deposited in the Herbarium of the Department of Botany, Postgraduate Jahan Zeb College, Swat, Pakistan.
Isolation of essential oil
Air-drying of the plant was performed in a shady place at room temperature and then crushed. The aerial parts of P. atriplicifolia. were submitted for 3 h to hydrodistillation using Clevenger-type apparatus. The obtained essential oil was dried over anhydrous sodium sulfate and filtered through cotton, and the oil was stored at 4°C until analysis.
Gas chromatography–mass spectrometry
The oil was analyzed by GC-MS using Perkin Elmer-Autosystem XL Gas Chromatograph and Perkin Elmer TurboMass mass spectrometry. PE-5ms (5% phenyl–95% methylpolysiloxane) column (20 × 0.18 mm Ø with 0.18-µm film thickness) was used with helium at 0.5 mL/min as the carrier gas; GC oven temperature was kept at 45°C for 2 min and programmed to 240°C at a rate of 6°C/min and kept constant at 240°C for 5 min. The split ratio was adjusted to 1:100, while the injection volume was 0.1 μL. Electron impact mass spectrometry (EI-MS) were taken at 70 eV ionization energy. Mass range was from m./z. 35 to 350 amu. Library research was carried out using NIST and Wiley's GC-MS Library of Essential Oil. The relative percentage amount of separated compounds was calculated from an ion chromatogram by a computerized integrator.
Antimicrobial activity tests
Microorganisms
Test organisms used in the current study were obtained from the Agriculture Research Service Culture Collection (NRRL), Peoria, Illinois, USA, the Biology Department of Eskişehir Osmangazi University, and the Biology Department of Anadolu University, Eskisehir, Turkey.
The bacterial strains tested were Bacillus cereus. NRRL B-3711, Bacillus cerus subsp. mycoides. NRRL B-4379, Bacillus subtilis. NRRL B-209, Micrococcus luteus. NRRL B-1018, Staphylococcus aureus. ATCC 25923, Staphylococcus epidermidis. NRRL B-4268, Streptococcus faecium. NRRL B-3502, Escherichia coli. ATCC 25922, Enterobacter aerogenes. NRRL B-3567, Pseudomonas aeruginosa. ATCC 10145, Klebsiella pneumoniae. (clinical isolate), and Yersinia enterocolitica.. Fungi used were Candida albicans. NRRL Y-12983, Aspergillus niger. ATCC 10549, Aspergillus flavus. NRRL 1957, Aspergillus fumigatus. NRRL 163, Aspergillus parasiticus. NRRL 465, and Geotricum candidum. (wild type).
Bacterial and fungal cultures of test organisms were maintained on nutrient agar (Merck, 1.05450, Schuchardt, OHG Germany) and malt extract agar (Merck, 1.05398) slants at 4°C, respectively and were subcultured in Petri dishes prior to use. Each combination of microorganisms and antibiotics was repeated three-times.
Agar diffusion methods
The agar diffusion method was used for antimicrobial activity. The antibacterial activity of the essential oil was tested against Gram-positive bacteria such as B. cereus., B. mycoides., B. subtilis., M. luteus., S. aureus., S. epidermidis.,and S. faecium. and Gram-negative bacteria such as E. coli., E. aerogenes., P. aeruginosa., Y. enterocolitica., and. K. pneumoniae. using Mueller-Hinton agar (MHA; Fluka, 70191, Steinheim, Germany). The antifungal activity of the essential oil was tested against two yeasts, C. albicans. and Saccharomyces cerevisiae., and five molds, A. niger., A. fumigatus., A. flavus., A. parasiticus., and G. candidum., using Sabouraud 4% glucose medium (SGM; Fluka, 84088).
Penicillin G (Sigma, 111H0079), tetracycline (Sigma, 20K1279, Steinheim, Germany), and cephotaxime (Fluka, 22128) were used as standards for antibacterial activity.
MHA and SGM, after autoclaving, were poured into Petri dishes to give a uniform depth of approximately 4 mm and were allowed to cool to room temperature. Test bacteria were transferred to tubes containing 4 to 5 mL of Mueller-Hinton broth (MHB) (Merck, 1.10293). The cultures were incubated at 35–37°C until visibly turbid. The density of these cultures was adjusted with sterile saline to that of the 0.5 McFarland standard (at 625 nm, 0.08–0.1 absorbance). Bacterial cultures adjusted to this standard contain approximately 108 CFU/mL. In contrast, to induce spores formation, molds were grown on potato dextrose agar (Merck, 1.10130) slants at 27°C for 5 to 7 days. Spore concentration was adjusted with sterile 0.1% Tween 80 (Merck, S26038) at 106 CFU/mL for each mold. The density of yeast culture was adjusted with sterile saline to that of the 0.5 McFarland and then diluted 107 CFU/mL.
The entire surface of the MHA and SGM plates was inoculated by streaking with a sterile swab dipped into adjusted suspension. Paper disks impregnated (Schleicher & Schuell, Ø6 mm) with 15 µL of the essential oil were placed on the surface of the inoculated agar plate. The plates were preincubated for 1 h at 4°C and incubated at 35°C for bacteria and at 27°C for fungi. After 18 h of incubation in the case of bacteria and 3 days of incubation for fungi, each plate was examined and the diameters of the zones of complete inhibition were measured, including the diameter of the disk (NCCLS M2-A4, 1990b).
Minimum inhibitory concentration
In determination of the minimal inhibitory concentration (MIC) of the essential oil, the agar-dilution method was employed. The essential oil was incorporated into the agar medium, with each plate containing a different concentration (10 to 640 µL/mL) of the agent. Meanwhile, one control without the essential oil was prepared.
The bacterial cultures containing approximately 108 CFU/mL were diluted 1:10 to obtain an inoculum concentration of 107 CFU/mL, after which 2 µL was inoculated on an agar surface area of 5 to 8 mm with standardized loops. After the spots had dried, the plates were inverted and incubated at 35°C (NCCLS M7-A2, 1990a; NCCLS M38-A, 2002). Molds and yeast suspensions of a density of 107 CFU/mL were inoculated on agar surface in the same way. MIC was recorded as the lowest concentration of antimicrobial agent that completely inhibited growth.
Mutagenicity and antimutagenicity tests
Microorganisms
Salmonella typhimurium. TA98 and TA100 were kindly provided by Dr. Bruce Ames (University of California, Berkeley, CA, USA).
Preparation of S9 fraction
Sprague-Dawley male rats were used for the preparation of liver S9 fraction. The animals were cared for according to the policies and principles established by the Animal Welfare Act and the NIH Guide for Care and Use of Laboratory Animals (publication no. 86–23). 3-Methylcholanthrene and phenobarbital were used for the induction of rat liver enzymes. 3-Methylcholanthrene was diluted in corn oil (125 mg/kg body weight) and injected intraperitoneally to each rat 5 days before sacrifice. In addition, phenobarbital was included into the drinking water (0.1% g/L) and administered for 5 days before sacrifice. During this period, the animals were kept in rooms illuminated from 0700 to 1900 h (12-h light/12-h dark cycle), maintained at 21–23°C, and had full access to pellet food and water ad libitum..
Preparation of the liver S9 fraction was based on the procedure described by Garner et al. (Citation1972). Phenobarbital and 3-methylcholanthrene were used as inducers for S9 fraction in this test. Consequently, two types of cytochromes (cyt P-450 and cyt P-448) were activated through the use of these two chemicals (Singer and Grunberger, Citation1983). The protein content of S9 fraction was found to be 12 mg/mL. During pilot tests, the S9 fraction was tested on S. typhimurium. with 2-aminofluorene, which is a positive mutagen in the presence of S9. The number of revertant colonies obtained with 2-aminofluorene was 25–30 times higher than that of the control group, and therefore, the protein content of S9 was sufficient for the metabolic activation system. Accordingly, the mutagenicity tests were undertaken using the S9 fraction with 12 mg/mL protein content.
Determination of cytotoxic dose of essential oil
The amounts of the essential oil used in the mutation assays were selected in the cytotoxicity assay. The rationale behind this test was to determine whether the test doses of the materials would have any cytotoxic effect: 0.1 mL of a suitable dilution of overnight bacterial culture was added to 2 mL top agar along with different concentrations of the tested chemicals. The top agar was poured onto nutrient agar plates, and assessment of cytotoxicity was performed after overnight incubation at 37°C (Dean et al., Citation1985).
Mutagenicity tests
The Salmonella. mutagenicity assay was carried out according to the method described by Maron and Ames (Citation1983). Non-cytotoxic doses of the tested sample were used in the experiments. We use DMSO as a solvent, and it is compatible with the Ames test (Maron and Ames, Citation1983). Oxoid nutrient broth no. 2 was used for overnight culture. For plate incorporation assays, 0.1 mL of bacterial tester strain, 0.5 mL of S9 mix if appropriate, and the sample to be tested was added to 2 mL of molten top agar. The contents were then mixed and poured onto agar plates. After 72 h of incubation, revertant colonies were counted by the method suggested by Claxton et al. (Citation1987). At least six plates were used for each dose with S9 fraction and at least 10 plates were used for each dose without S9 fraction. Sodium azide and daunomycin were used as positive mutagens for TA100 and TA98, respectively, without S9 fraction, and 2-aminofluorene was used for both strains with S9 fraction. The strains were then checked routinely for ampicillin resistance, ultraviolet-light sensitivity, crystal-violet sensitivity, histidine requirement, and spontaneous reversion rate. The materials were stored at − 80°C.
Antimutagenicity test
The antimutagenicity testing consisted of combining 0.1 mL of the bacterial culture of the tester strain, 0.1 mL of the test samples, and 0.1 mL of mutagen in soft agar poured onto a minimal agar plate. After incubation at 37°C for 48–72 h, revertant colonies were counted to determine the inhibitory effects, expressed as the inhibition rate. The tested substances were considered to possess antimutagenic activity, which was expressed with the positive rate: Inhibition rate % = [(A. − B.)/A.] × 100, where A. is positive revertant colonies, and B. is revertant colonies after adding the test samples (Diril et al., Citation1995). Without S9 fraction, sodium azide and daunomycin were used as positive mutagens for TA100 and TA98, respectively, and with S9 fraction, 2-aminofluorene was used as positive mutagen for both strains.
Statistical analysis
The data obtained from the experiments were analyzed with the SPSS 11.00 program. Significance levels sets at p < 0.05 evaluated by Games-Howell test were employed to determine the groups leading to significant differences and resulting in antimutagenic effects (Montgomery, Citation2002). The differences between the revertant colonies of the test groups and the control group were tested with Games-Howel test at 95% confidence level. Doses higher than the mean of the control group and consequent mutagenic condition were defined as “mutagenic,” whereas an increase in dose approaching to, but not reaching a twofold increase was defined as “weak mutagenic” (Claxton et al., Citation1987).
Results and Discussion
Chemical composition of the essential oil
Air-dried plant material was subjected to hydrodistillation using a Clevenger apparatus. The yields oil was 3.2% (v/w). The percentage compositions of the oil components are listed in . Eighteen components were detected in the oil of P. atriplicifolia. representing 96.06% of the total oil. The oil consisted of seven non-oxygenated monoterpenes (29.22%), three oxygenated monoterpenes (3.84%), one monoterpenoid ketone (28.91%), five sesquiterpenes of which one was oxygenated (30.57%), and two esters (3.52%). The main constituent was camphor (28.90%). Other important compounds were limonene (16.72%), α.-globulol (10.21%), trans.-caryophyllene (9.30%), and α.-humulene (9.25%). The essential oil also contained smaller percentages of (E.)-β.-ocimene (4.00%), α.-pinene (3.84%), camphene (3.76%), borneol (3.54%), bornyl acetate (2.07%), terpynil acetate (1.45%), T-cadinol (1.07%), (−)-caryophyllene oxide (0.74%), β.-pinene (0.57%), δ.-terpinene (0.33%), and linalool (0.30%). β.-myrcene and 1,8-cineole were trace amounts.
Table 1.. Chemical composition of Perovskia atriplicifolia..
Literature review showed variation between the chemical composition of Perovskia atriplicifolia and P. abrotanoides. oils (Younos et al., Citation1972). Furthermore, the chemical composition of the essential oil of Perovskia atriplicifolia. shows variability within the same species; it seems to depend on the genetic characteristics of the plant and on the conditions under which it has grown (Skocibusic et al., Citation2006). Even the composition of essential oils from a particular species of plant can differ between harvesting seasons and geographic sources (Burt, Citation2004).
Antimicrobial activity
The in vitro. antimicrobial activity of P. atriplicifolia. essential oil against the microorganisms employed and its activity potentials were qualitatively and quantitatively assessed by the presence or absence of inhibition zones, zone diameters, and MIC values. According to the results given in Tables and , the essential oil of the investigated species had significant in vitro. potential of antimicrobial activities against 10 bacteria, 4 molds, and a yeast species tested. The data obtained from the disk diffusion method indicated that the essential oil displayed a variable degree of antimicrobial activity on different tested strains. The data indicated that Gram-positive bacteria including B. subtilis, B. mycoides., and B. cereus. were the most sensitive strains tested to the oil of P. atriplicifolia. with the strongest inhibition zones (14 mm, 10 mm, 10 mm, respectively). The oil also exhibited high antibacterial activity against M. luteus.. Modest activities were observed against food–borne pathogen S. aureus. and other Gram-positive bacteria, S. epidermidis. and S. faecium. with inhibition zones of 8–9 mm. The Gram-negative bacteria tested were less susceptible than Gram-positive bacteria and displayed variable degree of susceptibility against the investigated oil. Modest activity was observed against Y. enterocolitica. and E. aerogenes.. The oil was inactive against Gram-negative bacteria P. aeruginosa. and K. pneumoniae., as the former is known to have a high level of intrinsic resistance to virtually all known antimicrobials and antibiotics because of a very restrictive outer membrane barrier, highly resistant even to synthetic drugs. The highest antifungal activity of this oil was observed against C. albicans. (14 mm) followed by A. flavus. (10 mm), G. candidum. (10 mm), A. fumigatus. and A. niger. (8 mm).
Table 2.. Antibacterial and antifungal activity of the essential oil.
Table 3.. Minimum inhibitory concentration (µL/mL) for the essential oil against bacterial strains.
The MIC of the essential oil ranged from 10 µL/mL to 160 µL/mL for most organisms (). MIC values were determined as 20 µL/mL against B. cereus., S. epidermidis., S. faecium;. and 10 µL/mL against B. mycoides., B. subtilis., M. luteus., and Y. enterocolitica.. Essential oil had no antimicrobial activity against P. aeruginosa. and K. pneumoniae. up to 640 µL/mL concentration. The essential oil of P. atriplicifolia. also showed strong activity against A. fumigatus., C. albicans., and A. flavus. at 40 µL/mL, 80 µL/Ml, and 80 µL/mL, respectively.
An important characteristic of essential oils and their components is their hydrophobicity, which enables them to partition in the lipids of the bacterial cell membrane and mitochondria, disturbing the structure and rendering them more permeable. Although a certain amount of leakage from bacterial cells may be tolerated without loss of viability, extensive loss of cell contents or the exit of critical molecules and ions will lead to death (Burt, Citation2004).
Also, these activities may be attributed to the presence of (E.)-β.-ocimene, β.-pinene, α.-pinene, 1,8-cineole, limonene, and camphene found in the essential oil of P. atriplicifolia.. Enantiomers of essential oil components have been shown to exhibit antimicrobial activity to different extents. Enantiomers of α.-pinene, β.-pinene, and limonene have a strong antibacterial activity (Lis-Balchin et al., 1999). It has been demonstrated that β.-pinene and α.-pinene are able to destroy cellular integrity and thereby inhibit respiration and ion transport processes (Uribe et al., Citation1985; Knoblock et al., Citation1988; Burt, Citation2004). They also increase the membrane permeability in yeast cell and isolated mitochondria (Andrews et al., Citation1980; Uribe et al., Citation1985). This is strongly supported by the study on the effects of different essential oil components on outer membrane permeability in Gram-negative bacteria (Helander et al., Citation1998). The possible mechanisms of other essential oil components such as trans.-caryophyllene, limonene, camphene, bornyl acetate, and α.-humulene have not yet been elucidated (Magwa et al., Citation2006).
Gram-positive bacteria are known to be more susceptible to essential oils than Gram-negative bacteria (Burt, Citation2004). The finding that E. coli. was least susceptible to the essential oil of P. atriplicifolia. is in accordance with previous studies. The weak antibacterial activity against Gram-negative bacteria was ascribed to the presence of an outer membrane, which possessed hydrophilic polysaccharide chains as a barrier to hydrophobic essential oils. Accordingly, a high degree of susceptibility of P. aeruginosa. and K. pneumonia. was not expected.
Mutagenic and antimutagenic activity
The mutagenic and antimutagenic activities of P. atriplicifolia. oil were evaluated in the Salmonella./microsome test system using Salmonella typhimurium. TA98 and TA100. The results are shown in . All concentrations of the essential oil did not show any mutagenic effects on the strains (p < 0.05).
Table 4.. Mutagenic and antimutagenic activity data of the essential oil in Salmonella. plate incorporation test using TA98 and TA100 with or without S9.
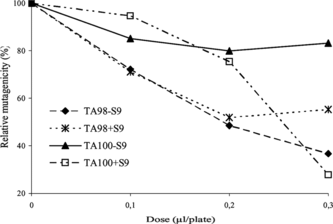
The results of antimutagenic effects of the essential oil are presented in . as plots of the percentage of the remaining mutagenicity. Antimutagenic activity of the oil was the strongest in the strain TA100 against 2-AF with S9 fraction (72%). Inhibition of mutagenicity was around 63.3% against daunomycin for TA98 without S9 fraction and 44.7% against 2-AF for TA98 with S9 fraction (p < 0.05).
Figure 1 Inhibitory effect of Perovskia atriplicifolia. essential oil against the mutagenicity of daunomycin (6 µg/plate), sodium azide (1.5 µg/plate), 2-aminofluorene (10 µg/plate) to S. typhimurium. TA98 and TA100 with and without S9 fraction.
Camphor and limonene were the major constituents of the P. atriplicifolia. oil studied. Gomes-Carneiro et al. (1998) investigated the mutagenic activity of camphor in the same assay and reported that no mutagenic effects were found. In another study, it was also reported that there was no mutagenic activity induced by 4-methylbenzylidene camphor employing the same test (Utesch & Splittgerber, Citation1996).
No mutagenic activity was observed for limonene, the second highest concentration found in the essential oil (Watabe et al., Citation1980; Haworth et al., Citation1983). Furthermore, another study stressed the high antimutagenic activity of d-limonene (Higashimoto et al., Citation1998).
1,8-Cineol and (−)-caryophyllene oxide were also present in the P. atriplicifolia. oil but at a lower concentration. No mutagenic effects were reported with these compounds in the same assay (Gomes-Carneiro et al., 1998; Padilha de Paula et al., 2003). Furthermore, Lee et al. (Citation1995) reported that caryophyllene oxide reduced mutagenicity of aflotoxin B-1 for S. typhymurium. TA98 and TA100 to 89% and 71%, respectively.
To our knowledge, the biological activities of P. atriplicifolia. has not been reported before, and therefore our results can be evaluated as the first report about the antimicrobial, mutagenic, and antimutagenic properties with respect to the chemical composition. The results of this study suggest that the essential oil from P. atriplicifolia. has potential as an antibacterial, antifungal, and antimutagenic agent and may be useful in the food, pharmaceutical, and cosmetic industries. Further research is needed in order to obtain information regarding the practical effectiveness of the essential oil.
Acknowledgment
The authors are grateful to Dr. İsmail Dityakat, of TÜBİTAK-MAM, Turkey, and Dr. Nuran Diril, of Hacettepe University, Turkey.
References
- Andrews RE, Parks LW, Spence KD (1980): Some effects of Douglas fir terpenes on certain microorganism. Appl Environ Microb 40: 301–304.
- Burt S (2004): Essential oils: Their antibacterial properties and potential application in foods—a review. Int J Food Microb 94: 223–253.
- Claxton LD, Allen J, Auletta A, Mortelmans K, Nestmann E,Zeiger E (1987): Guide for the Salmonella typhimurium./mammalian microsome test. Mutat Res 189: 83–91.
- Dabiri M, Sefidkon F (2001): Analysis of the essential oil from aerial parts of Perovskia atriplicifolia. Benth. at different stages of plant growth. Flavour Frag J 16: 435–438.
- Dean BJ, Brooks TM, Hodson-Walker G, Huston DH, (1985): Genetic toxicology testing of 41 industrial chemicals. Mutat Res 153: 57–77.
- Diril N, Öksüzoğlu E, Öğüş A (1995): Investigation of cytotoxic, mutagenic and antimutagenic effects of ascorbic acid, Cu (II) and aspirin by Salmonella mutagenicity assay. Toxicol Envirol Chem 52: 215–220.
- Garner RC, Miller EC, Miller JA (1972): Liver microsomal metabolism of aflatoxin B1 to reactive derivative toxic to Salmonella typhimurium. TA1530. Cancer Res 32: 2058–2066.
- Gomes-Carneiro MR, Felzenszwalb I, Paumgartten FJR (1998): Mutagenicity testing of (racemic)-camphor, 1,8-cineole, citral, citronellal, (-)-menthol and terpineol with the Salmonella./microsome assay. Mutat Res 416: 129–136.
- Haworth S, Lawlor T, Mortelmans K, Speck W, Zeiger E (1983): Salmonella. mutagenicity test results for 250 chemicals. Environ Mutagen Supp 1: 3–142.
- Helander IM, Alkomi HL, Latva-Kala K, Mattilla-Sandholm T, Pol I, Smid EJ, Gorris LGM, Von Wright A (1998): Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46: 3590–3595.
- Higashimoto M, Yamato H, Kinouchi T, Ohnishi Y (1998): Inhibitory effects of citrus fruits on the mutagenicity of 1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-carboxylic acid treated with nitrite in the presence of ethanol. Mutat Res 415: 219–226.
- Ipek E, Zeytinoglu H, Okay S, Tuylu, BA, Kurkcuoglu M, Baser KHC (2005): Genotoxicity and antigenotoxicity of Origanum. oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem 93: 551–556.
- Jassbi AR, Ahmad VU, Tareen R (1999): Constituents of the essential oil of Perovskia atriplicifolia. Benth. Flavour Frag J 14: 38–40.
- Knoblock K, Pauli A, Iberl B, Weis N, Weigand H (1988): Antibacterial activity and antifungal properties of essential oil components. J Essent Oil Res 1: 119–128.
- Lee JM, Lee EJ, Bahn KN, Kim JO, Ha YL (1995): Potent antimutagenic activity of caryophyllene oxide for aflatoxin B-1 (AFB-1) and 2-amino-3-methylimidazo (4,5-f.) quinoline. Agric Chem Biotech 38: 468–472.
- Lis-Balchin M, Ochoka RJ, Deans SG, Asztemborska M, Hart S (1999): Differences in bioactivity between the enantiomers of α.-pinene. J Essent Oil Res 11: 393–397.
- Magwa ML, Gundidza M, Gweru N, Humphrey G (2006): Chemical composition and biological activities of essential oil from the leaves of Sesuvium portulacastrum.. J Ethnopharmacol 103: 85–89.
- Maron DM, Ames BN (1983): Revised method for the Salmonella. mutagenicity test. Mutat Res 113: 173–215.
- Montgomery DC (2002): Design and Analysis of Experiments New York, Wiley, p. 684.
- Mucciarelli M, Maffei M, Sacco T (1993): Glandular trichome essential oils of Perovskia atriplicifolia. Benth. and P. scrophulariaefolia. Bunge. Rivista Italiana Eppos. 9: 3–7.
- National Committee for Clinical Laboratory Standards (1990a): Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Second Edition. Approved Standard NCCLS Document M7-A2, Vol. 10, No.8. Villanova, PA, NCCLS.
- National Committee for Clinical Laboratory Standards (1990b): Performance Standards for Antimicrobial Disk Susceptibility Tests–Fourth Edition. Approved Standard NCCLS Document M2-A4, Vol. 10, No. 7. Villanova, PA, NCCLS.
- National Committee for Clinical Laboratory Standards (2002): Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi Approved Standard, NCCLS Document M38-A, Vol. 22, No. 16. Villanova, PA, NCCLS.
- Padilha de Paula J, Gomes-Carneiro MR, Paumgartten FJR (2003): Chemical composition, toxicity and mosquito repellency of Ocimum selloi. oil. J Ethnopharmacol 88: 253–260.
- Pourmortazavi SM (2003): Supercritical carbon dioxide extraction of essential oils from Perovskia atriplicifolia. Benth. J Agric Food Chem 57: 5414–5419.
- Rao MG (1926): Essential oil from the flowerheads of Perovskia atriplicifolia. Benth. Q J Ind Chem Soc 3: 141–147.
- Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M, Bruni R (2005): Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem 91: 621–632.
- Sefidkon F, Ahmadi L, Mirza M (1997): Volatile components of Perovskia atriplicifolia. Benth. J Essent Oil Res 9: 101–103.
- Singer B, Grunberger D (1983): Molecular Biology of Mutagen and Carcinogens. New York, Plenum Press, p. 347.
- Skocibusic M, Bezic N, Dunkic V (2006): Phytochemical composition and antimicrobial activities of the essential oils from Satureja subspicata. Vis. growing in Croatia. Food Chem 96: 20–28.
- Uribe S, Ramirez T, Pena A (1985): Effects of β.-pinene on yeast membrane functions. J Bacteriol 161: 195–200.
- Utesch D, Splittgerber J (1996): Bacterial photomutagenicity testing: Distinction between direct, enzyme-mediated and light-induced events. Mutat Res 361: 41–48.
- Watabe T, Hiratsuka A, Isobe M, Ozawa N (1980): Metabolism of d-limonene by hepatic microsomes to non-mutagenic epoxides toward Salmonella typhimurium.. Biochem Pharmacol 29: 1068–1071.
- Younos C, Lorrian M, Pelt JM (1972): Essential oils from two Perovskia species, P. abrotanoides. and P. atriplicifolia.. Plant Med Phytother 6: 178–182.
