Abstract
Mormodica involucrata. E. Mey. ex Sond (Curcubitaceae; balsamina) is used in Swaziland traditional medicine for treating diabetes, intestinal disorders, and also for culinary purposes. At concentrations of 150 and 300 mg/mL, the extract significantly inhibited acetylcholine-induced contractions of the rat stomach strip (RSS) by 15 ± 1.0% and 25 ± 1.5%, respectively (p < 0.05, n = 6). The action of the extract was reversed by mevinphos 10−9 M, an organophosphate anticholinesterase, and potentiated acetylcholine-induced contractile effects on RSS. In the analgesic study, the extract at 55–75 mg/kg inhibited acetic acid–induced writhing and induced sleep in mice, and higher concentrations of the extract in the range 350–480 mg/kg caused death within 1 h of i.p. administration in 20% and 100%, respectively (p < 0.05, n = 10 per treatment). These results show that M. involucrate. possesses antispasmodic, analgesic, and sedative effects in rodents.
Introduction
In Swaziland, a southern African country, the plant Mormodica involucrata. E. May ex Sond (Curcubitaceae), commonly known as “inkakha,” is widely used for diabetes and medicinal remedies as well as for culinary purposes. One of the many uses of the plant is for the treatment of pain, and we have recently reported an analgesic effect of another species of the plant (Aziba et al., Citation2001).
However, there are many other common uses of the plant for treatment of various ailments (Oliver Bever, Citation1986; Daziel, Citation1987; Iwu, Citation1993). A decoction of the leaves is used as a laxative for the relief of stomachache, an anthelmintic, and for the treatment of fever (Iwu, Citation1993). The current study was undertaken to further assess its contractile and receptor effects with mevinphos, an anticholinesterase, and to assess its analgesic and sedative effects because of signs of sleep and death observed in our previous studies (unpublished observation).
Plant material
Mormodica involucrata. was obtained in November 2004 from fields near the village of Kwaluseni next to the University of Swaziland. Mr. Gideon Dlamini, a curator at Malkerns Research Station, confirmed the identity of the plant. A voucher specimen with number RT 057 was deposited in the station herbarium.
Extraction of. Mormodica involucrata
Fresh leaves (100 g) were macerated in water for 24 h. Solvent elimination was carried out under reduced pressure, which yielded a light greenish semisolid compound.
Phytochemical screening.
A phytochemical analysis of the extract of M. involucrata. was conducted according to the method of Trease and Evans (Citation1983). Positive test results were obtained for saponins, glycosides, tannins, and flavonoids.
Animals.
Male Wistar rats (200–250 g) and Swiss mice of either sex (25–28 g) were raised in the experimental animal house of the Swaziland Institute of Medicinal Plant Research under standard environmental conditions. All animals had free access to rodent pellets and water.
Experimental procedure (in vitro.)
The rat stomach strip (RSS) was excised and placed in a dish containing Tyrode salt solution at 37°C, prepared according to Vane (Citation1957). The Tyrode solution was of the following composition: NaCI (137 mM), KCl (2.8 mM), CaC12 · 2H2O (1.8 mM), MgC12 · 6H2O (1.3 mM), NaHCO3(11.0 mM), and glucose (2 mM). Circular muscle strip was prepared and suspended in a 15-mL organ bath containing Tyrode solution, using a force displacement transducer (7010 Ugo Basil, Italy) under a resting tension of 0.5 g. The preparations were equilibrated for 1 h in a relaxed condition under aeration. After equilibration, the contractile responses were recorded isometrically and on a two-channel recorder (Gemini 7070).
In vitro study.
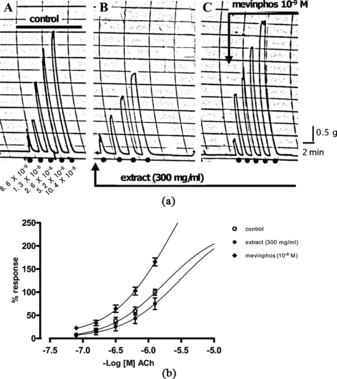
Dose-effect relationship was established using acetylcholine (ACH) concentrations, ranging from 6.6 × 10−9 M to 10.4 × 10−8 M), after which the strip was equilibrated in extract (300 mg/mL) for 5 min. The tissue was again stimulated with same doses of Ach; thereafter suspended in mevinphos 10−9 M (an irreversible anticholinesterase was added for 3 min in the presence of the extract, and the tissue was again stimulated with same doses of Ach; contractions were recorded (a).
Figure 1 (a) Tracings show the inhibitory and reversal effects of extract (300 mg/mL) and mevinphos (10−9M), respectively, on Ach-induced contractions of RSS. Control responses to ach (6.6 × 10−9 M to 10.2 × 10−8 M) are shown in A., extract inhibited responses to Ach (1.3 × 10−8 M to 10.2 × 10−9 M) are shown in B., and reversed effects by mevinphos to Ach (same concentrations as controls) are shown in C.. (b) The inhibitory effects of extract (300 mg/mL) and the potentiation effects of mevinphos (10−9 M) on Ach-induced (6.6 × 10−9 M to 10.2 × 10−8 M) contractions of RSS. Data are shown as the mean (±SE) responses (n = 6 per treatment). Responses from both treatments, extract and mevinphos, were significantly different than those of controls (paired t.-tests, p < 0.05).
In vivo studies.
Analgesic activity of the extract (55–75 mg/mL) was evaluated on acetic acid–induced writhing in mice by the method of Whittle (Citation1964). Each mouse (n = 5 per treatment) was given an 0.4 mL intraperitoneal (i.p.) injection containing a 1% (v/v) solution of acetic acid in saline solution. Controls received an equal volume of saline. Thirty minutes prior to the pain-inducing injections, some mice also received a treatment of either plant extract (55–75 mg/kg) or paracetamol (10 mg/kg) as a reference drug. The numbers of induced writhings were recorded by stopwatch for 20 min after i.p. injection.
Concentrations of extract (55–75 mg/kg) were administered to a group of mice (n = 5 per treatment). Sleep induced within 30 min of i.p. extract injection was determined to be the end point. If sleep occurred within 30 min of the injection and lasted for 1½ h, it was considered a positive response, otherwise the response was negative. Higher concentrations (350–480 mg/kg) similarly administered to mice (n = 10 per treatment) increasingly caused death within 1½ h of injection.
Statistical analysis
Statistical analyses of analgesic effects were performed using analysis of variance (ANOVA) followed by the Dunnett's post hoc. procedure. Analyses of Ach-contractile responses were performed using the Student's paired t.-test. Statistical significance was considered to be p < 0.05. All data are expressed as mean ± standard error (SE).
Results and Discussion
Aqueous extract of Mormodica involucrata. significantly inhibited the Ach-induced contractile response of the RSS (a), indicating an antispasmodic-like action (Aziba et al., Citation2001). Ach-induced contractions in RSS involve two different mechanisms coupled to muscarinic receptors. One is the activation of nonselective ion channels in the plasma membrane due to membrane depolarization. The membrane potential stimulates Ca2+ influx through voltage-operated channels (Bolton, Citation1979). The other mechanism is the activation, influx, or release of intracellular calcium (Kobayashi et al., Citation1989). The reversal and potentiating action of mevinphos (b), an irreversible anticholinesterase, indicated an indirect action on Ach by inhibiting the effect of cholinesterases. This action confirms properties of anticholinesterase on Ach-induced contractions.
The phytochemical analysis showed the presence of tannins, saponins, glycosides, and flavonoids (Trease & Evans, Citation1983). These constituents are known to be bioactive agents. The reduction of writhing counts is indicative of the analgesic potential of the extract (), and the sleep and death observed is indicative of sedative and lethal effects of the extract (Tables and ), respectively. It also shows a dual action of the extract. These findings suggest the need for further studies on the curative lethal doses and the safety margin for the use of the extract in traditional medicine practice.
Table 1.. The percent inhibitory analgesic properties of Mormodica involucrata. and paracetamol on acetic acid writhing response in mice. (Writhing numbers are expressed as mean ± SE, n = 5 per treatment.)
Table 2.. The sedative properties of extract Mormodica involucrata. (55–75 mg/mL) on sleep in mice (n = 10 per treatment)
Table 3.. Lethality of high doses (350–480 mg/kg) of Mormodica involucrata. treatments in mice (n = 10 per treatment)
Acknowledgments
The authors are grateful to Prof. O.O.G. Amusan for his support and Mr. Gugu Mavuso for technical assistance.
References
- Aziba PI, Ekor M, Adedeji A (2001): Pharmacological screening of a methanol extract from Mormodica. in rodents. Pharm Biol 39: 305–307.
- Bolton TB (1979): Mechanism of action of transmitter and other substances on smooth muscle. Physiol Rev 59: 607–718.
- Daziel JM (1987): The Useful Plants of Tropical West Africa. London, Crown Agents, p. 31.
- Iwu MM (1993): Handbook of Africa Medicinal Plants. Boca Raton, CRCS Press, p. 57.
- Kobayashi S, Kitazawa I, Somlyo AV, Trentham DR, Somlyo AP (1989): Heparin-sensitive inositol trisphosphate signaling and the role of G-protein in Ca2+ release and contractile regulation in smooth muscle. Prog Clin Biol Res 327: 167–182.
- Komori S, Bolton TB (1991): The involvement of rynodine receptors in muscarinic receptor mediated current. J Physiol 433: 495–517.
- Oliver Beaver B (1986): Medicinal Plants in Tropical. West Africa, London, Cambridge University Press, p. 108.
- Trease GE, Evans MC (1983): Textbook of Pharmacology. London, Valliere Tindal, pp. 343–383.
- Vane JR (1957): A sensitive method for assay of 5-hydroxytryptamine. Br J Pharmacol Exp Ther 218: 459–464.
- Whittle BA (1964): The use of changes in capillary permeability in mice to distinguish between, narcotic and narcotic analgesics. Br J Pharmacol Chem 22: 246–263.
