Abstract
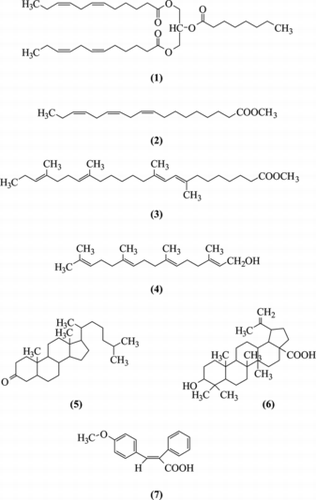
Seven constituents were isolated from the stems and leaves of Croton lobatus. L. (Euphorbiaceae), a medicinal plant used in western Africa in traditional folk medicine to cure malaria, pregnancy troubles, and dysentery. Their structures were elucidated by spectroscopic methods. The compounds identified were 3-[(6Z.,9Z.)dodeca-6,9-dienoyloxy]-2-octanoyloxypropyl(6Z.,9Z.)dodeca-6,9-dienoate (1) for the first time and six known compounds: (Z.,Z.,Z.)-9,12,15-octadecatrienoic acid methyl ester (2), 8,11,17,21-tetramethyl-(E.,E.,E.,E.)-8,10,17,21-tetraentetracosanoic acid (3), geranylgeraniol (4), cholestan-3-one (5), betulinic acid (6), and (E.)-3-(4-methoxy-phenyl)-2-phenyl-acrylic acid (7). From the seven compounds, (4) and (6) showed the best antiplasmodial activity in vitro. on Plasmodium falciparum. K1 chloroquine-resistant strain, with IC50 values (µg/mL) of 1.07 and 1.45 mg/mL, respectively, while compounds (2), (3), and (7) showed IC50 below 5 in the same assay. Cytotoxicity of the most active compounds was evaluated on L6 murine myoblast cells. Geranylgeraniol (4) showed good selectivity with an SI value (SI = ratio of cytotoxicity to biological activity) over 25.
Introduction
Malaria is one of the most important health problems in the world and is responsible for more than 1 million deaths per year in tropical and subtropical areas in more than 100 countries. The increased threat represented by malaria comes from the resistance of the parasite to chloroquine, the cheapest and previously very effective antimalarial drug, and to pyrimethamine in the past decade (Wellems & Plowe, Citation2001). Therefore, there is an urgent need to discover new antimalarial agents.
History shows that biodiversity and traditional medicine knowledge are useful to open new venues in the field of antimalarial therapy, as was the case for artemisinin (Meshnick, Citation2002). We focused our interest on Croton lobatus. L. (Euphorbiaceae), a shrub growing wild in west tropical Africa, which is used traditionally in various African countries to cure several diseases, and in particular malaria. A first study showed good in vitro. antiplasmodial activity of the aerial parts and root crude extracts of this species (Weniger et al., Citation2004). We present here a phytochemical investigation of the stems and leaves of this medicinal plant that led to the isolation and structural determination of seven compounds some of them with interesting antiplasmodial activity.
Materials and Methods
General experimental procedures
Melting points were determined by using a Büchi melting point B-545 apparatus. UV spectra were obtained on a Hewlett Packard 8452A diode array spectrometer (Hewlett Packard, Agilent Technologies, Massy, France and Bruker, Wissembourg, France). IR spectra were performed using a FT-IR Bruker IFS25 spectrometer. 1H and 13C NMR spectra were recorded with Bruker Avance 300 MHz, and 1H-1H correlation spectroscopy (COSY), distortionless enhancement by polarization transfer (DEPT), 2D- heteronuclear single quantum correlation (HSQC), and 2D-heteronuclear multiple bond correlation (HMBC) spectra with 400 MHz spectrometers. Chemical shift values are reported in ppm with reference to TMS. Coupling constants are given in hertz. High resolution electron ionization mass spectrometry (HREIMS) were obtained on an Autospec Micromass (70 eV) instrument (Hewlett Packard). Column chromatography was performed with silica gel 60 (Merck, 43–60 mesh) and TLC with silica gel F254.
Plant material
C. lobatus. L. (Euphorbiaceae) stems and leaves were collected in the suburbs of Abidjan, Ivory Coast, during October 2003. A voucher specimen (no. 8950) was deposited in the Herbarium of the Botanical Garden of Cocody University (Abidjan, Ivory Coast).
Extraction and isolation
The air-dried leaves and stems of C. lobatus. were ground mechanically. A total amount of 2250 g was obtained and was divided in two portions. The first portion of 958 g was extracted under reflux overnight, using the ternary solvent methanol–methylene chloride–acetic acid (4:5:1). Acetic acid is a weak carboxylic acid, which increased the polarity of the solvent extraction, but under these conditions, no structure modifications were observed. The filtrates were combined and the organic solvents were removed under reduced pressure. A crude extract (E1) of 123 g was isolated. E1 62.6 g was chromatographed on a silica gel column (silica gel 60, Merck, 43–60 mesh) using an elution gradient consisting of cyclohexane–methylene chloride (from 4:1 to 3:2) yielding four compounds: 1 (24 mg), 2 (22 mg), 3 (25 mg), and 4 (62 mg) ().
The second portion of 1292 g was extracted by maceration at room temperature in ethanol (3 × 5 L) for 12 h. The combined extracts were concentrated under reduced pressure until a volume of 500 mL, which was washed with cyclohexane (3 × 500 mL). The combined extracts were evaporated under vacuum to dryness to give a dark-green gum (30.45 g). The gum was further washed with ethyl acetate–aqueous sulfuric acid 10% (49:1) (3 × 500 mL). The different extracts were evaporated under reduced pressure to dryness to give a second fraction (15.23 g), which was subjected to silica gel column chromatography using a gradient mixture of cyclohexane–ethyl acetate and ethyl acetate–methanol as eluant. The purification process yielded three compounds: 5 (40 mg), 6 (225 mg), and 7 (10 mg) (). The structures of the isolated compounds were determined by 1D and 2D NMR techniques, mass spectra, and by comparison with spectroscopic data published in the literature.
Assay for antiplasmodial activity
The antiplasmodial activity was evaluated against the parasite Plasmodium falciparum. (K1 strain, multidrug-resistant pyrimethamine and chloroquine-resistant strain), which was cultured continuously according to a described method (Trager & Jensen, Citation1976). Quantitative assessment of antimalarial activity in vitro. was determined by the microculture radioisotope technique (Ridley, Citation2002). The assay uses the uptake of [3H]hypoxanthine (Amersham International, Buckinghamshire, UK) by parasites as an indicator of viability. Initial concentration of each compound was 30 µg/mL diluted with twofold dilutions to make seven concentrations, the lowest being 0.47 µg/mL. After 48 h incubation of the parasites with compound solution at 37°C, [3H]hypoxanthine was added to each well and the incubation was continued for another 24 h at the same temperature. The inhibitory concentration (IC50) represents the concentration that causes 50% reduction in parasite growth as indicated by the in vitro. incorporation of [3H]hypoxanthine by P. falciparum.. In this test system, IC50 values of 0.043 µg/mL and 0.0015 µg/mL were observed for the standard compounds chloroquine and artemisinin, respectively.
Assay for cytotoxicity
Cytotoxicity was evaluated according to a described method (Ahmed et al., Citation1994). L6 myoblasts of rat skeletal muscles were cultivated in 96-well COSTAR plates in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum. All cells were incubated in an atmosphere containing 5% CO2 at 37°C for 72 h. Cytotoxicity was calculated by using the same concentration as for antiplasmodial activity. After addition of Alamar blue as viability indicator and 2 h further incubation, fluorescence was evaluated by using an excited wavelength of 536 nm and emission recorded at 588 nm. IC50 values were calculated from sigmoid inhibition curves.
Results and Discussion
The phytochemical investigation of the stems and leaves of Croton lobatus. led to the isolation and characterization of seven compounds (see ). 3-[(6Z.,9Z.)Dodeca-6,9-dienoyloxy]-2-octanoyloxypropyl(6Z.,9Z.)dodeca-6,9-dienoate (1) is a novel compound, which was further called lobaceride.
Lobaceride (1): slight-yellow oil; UV (MeOH) λmax (log ε) 220 (4.20), 310 (3.80); IR (film) νmax 2918, 2848, 1742 (br) cm−1; 1H NMR, see ; 13C NMR, see ; ESIMS: m./z. 575 (M + H); HREIMS m./z. 574.42333 (calcd. 574.42334); Anal. C 73.13%, H 10.21%, calcd. for C35H58O6 C 73.17%, H 10.10%.
Table 1. 1H and 13C NMR spectral data assignments for lobaceride (1) in CDCl3 (13C at 75.4778 MHz and 1H at 300 MHz). Chemical shifts are given in ppm, coupling constant J. values (in parentheses) are in Hertz.
Six other known compounds were identified as (Z.,Z.,Z.)-9,12,15-octadecatrienoic acid methyl ester (2) (Mancini et al., Citation1998), 8,11,17,21-tetramethyl-(E.,E.,E.,E.)-8,10,17,21-tetraentetracosanoic acid (3) (Cavell & MacMillan, 1967), geranylgeraniol (4) (Coates et al., Citation1978), cholestan-3-one (5) (Seldes et al., 1990), betulinic acid (6) (Ikuta & Itokawa, Citation1988), and (E.)-3-(4-methoxy-phenyl)-2-phenyl-acrylic acid (7) (Halton et al., Citation1984). Their structures were elucidated on the basis of NMR and mass spectral data in the literature.
The antiplasmodial activities of the different compounds were evaluated in vitro. on the multidrug–resistant Plasmodium falciparum. K1 strain. Results are summarized in . Two of the isolated compounds, geranylgeraniol (4) and betulinic acid (6) showed good antiplasmodial activity with IC50 below 2 µg/mL. Three other constituents, namely (Z.,Z.,Z.)-9,12,15-octadecatrienoic acid methyl ester (2), 8,11,17,21-tetramethyl-(E.,E.,E.,E.)-8,10,17,21-tetraentetracosanoic acid (3) and (E.)-3-(4-methoxy-phenyl)-2-phenyl-acrylic acid (7) showed IC50 below 5 µg/mL.
Table 2. Antiplasmodial activity, cytotoxicity, and selectivity indices of isolated constitutents.
In order to clarify the potential of the tested compounds for therapeutic use, we determined the cytotoxicity of the most active compounds on the L6 murine myoblast cell line (see ). All values are expressed as IC50 in µg/mL, and for the most active extracts (IC50 < 2 µg/mL), IC50 values are means ± SD (n = 2). Geranylgeraniol (4) showed the best selectivity index (SI = ratio of cytotoxicity to biological activity) with a SI value over 25. It is generally considered that biological efficacy is not due to in vitro. cytotoxicity when SI≥10.
The Croton. genus contains 800 species, some of which are known to contain bioactive or toxic constituents (Araújo-Júnior et al., Citation2004). For instance, three 8,9-secokaurane diterpenes isolated from C. kongensis. were shown to possess in vitro. antimalarial activity against the Plasmodium falciparum. K1 strain, with IC50 values in 1.0–2.8 µg/mL range (Thongtan et al., Citation2003). C. oblongifolius. and C. zambesicus. showed moderate in vitro. cytotoxicity against human tumor cell cultures (Roengsumran et al., Citation1999; Block et al., Citation2004). Betulinic acid and its derivatives are well-known for their in vitro. anti HIV-1 activity and their specific cytotoxicity against a variety of tumor cell lines (Zuco et al., Citation2002; Aiken & Chen, Citation2005). Geranylgeraniol is known to induce apoptosis in leukemia cell lines in a way that differs from usual mechanisms of chemotherapeutic drugs (Masuda et al., Citation2000).
Acknowledgments
The authors thank the Academic Authority of Ivory Coast for the scholarship to B. Attioua. They are also grateful to A.U.F. (Agence Universitaire de la Francophonie-PCSI 6313PS562) for financial support in this research program.
References
- Ahmed SA, Gogal RM, Walsh JE (1994): A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]-thymidine incorporation assay. J Immunol Methods 170: 211–224.
- Aiken C, Chen CH (2005): Betulinic acid derivatives as HIV-1 antivirals. Trends Mol Med 11: 31–36.
- Araújo-Júnior VT, Silva MS, Leitaˇo da-Cunha EV, Agra MF, Silva-Filho RN, Barbosa-Filho JM, Braz-Filho R (2004): Alkaloids and diterpenes from Croton moritibensis.. Pharm Biol 42: 62–67.
- Block S, Baccelli C, Tinant B, Van Meervelt L, Rozenberg R, Habib Jiwan JL, Llabrès G, De Pauw-Gillet MC, Quetin-Leclercq J (2004): Diterpenes from the leaves of Croton zambesicus.. Phytochemistry 65: 1165–1171.
- Cavell BD, MacMillan J (1967): Isolation of (-)-kaur-16-en-19-oic acid from the mycelium of Gibberella fujikuroi.. Phytochemistry 6: 1151–1154.
- Coates RM, Ley DA, Cavender PL (1978): Synthesis and carbon-13 nuclear magnetic resonance spectra of all-trans.-geranylgeraniol and its nor analogues. J Org Chem 43: 4915–4922.
- Halton B, Maidment AI, Officer DL, Warnes JM (1984): The oxidative conversion of (E.)-α-(arylmethylene)-benzeneacetates into substituted phenanthrenes: The propitious use of boron trifluoride with vanadium trifluoride oxide. Aust J Chem 37: 2119–2128.
- Ikuta A, Itokawa H (1988): Triterpenoids of Paeonia japonica. callus tissue. Phytochemistry 27: 2813–2815.
- Mancini I, Guella G, Defant A, Candenas ML, Armesto CP, Depentori D, Pietra F (1998): Polar metabolites of the tropical green seaweed Caulerpa taxifolia. which is spreading in the Mediterranean sea: Glycoglycerolipids and stable enols (α-keto esters). Helv Chim Acta 81: 1681–1691.
- Masuda Y, Nakaya M, Aiuchi T, Hashimoto S, Nakajo S, Nakaya K (2000): The mechanism of geranylgeraniol-induced apoptosis involves activation, by a caspase-3-like protease, of a c-Jun N-terminal kinase signaling cascade and differs from mechanisms of apoptosis induced by conventional chemotherapeutic drugs. Leuk Res 24: 937–950.
- Meshnick SR (2002): Artemisinin: Mechanisms of action, resistance and toxicity. Int J Parasitol 32: 1655–1660.
- Ridley RG (2002): Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415: 686–693.
- Roengsumran S, Singtothong P, Pudhom K, Ngamrochanavanich N, Petsom A, Chaichantipyuth C (1999): Neocrotocembranal from Croton oblongifolius.. J Nat Prod 62: 1163–1164.
- Seldes AM, Deluca ME, Gros EG, Rovirosa J, San-Martin A, Darias J (1990): Steroids from aquatic organisms. New sterols from the antarctic sponge Artemisina apollinis.. Z Naturforsch 45B: 83–86.
- Thongtan J, Kittakoop P, Ruangrungsi N, Saenboonrueng J, Thebtaranonth Y (2003): New antimycobacterial and antimalarial 8,9-secokaurane diterpenes from Croton kongensis.. J Nat Prod 66: 868–870.
- Trager W, Jensen JB (1976): Human malaria parasites in continuous culture. Science 193: 673–675.
- Wellems T, Plowe C (2001): Chloroquine-resistant malaria. J Infect Dis 184: 770–776.
- Weniger B, Lagnika L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, Brun R, Anton R, Sanni A (2004): Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro. antiplasmodial activity. J Ethnopharm 90: 279–284.
- Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Gambacorti-Passerini C, Formelli F (2002): Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 175: 17–25.