Abstract
The lignan-rich petroleum ether extract and the isolated lignans, simplexolin and sesamolin, of Justicia simplex. D. Don (Acanthaceae) were tested for protective effect on CCl4-induced hepatotoxicity in rats. The induced CCl4 toxicity elevated the levels of marker enzymes alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and stimulated lipid peroxidation along with decrease in triglycerides and glutathione content. The pretreatment with the extract and the isolated lignans at the dose of 100 mg/kg p.o. and 10 mg/kg p.o., respectively, normalized these toxic levels. The effect of the isolated lignans was comparable with the standard (Nirocil). This showed the hepatoprotective effect of petroleum other extract and lignans of J. simplex..
Introduction
Justicia simplex. D. Don (Acanthaceae) is a well-known traditional medicinal plant, native to the Western Himalayas, and used as an antifatigue and stimulating agent. Our earlier studies revealed the presence of lignans and triterpenoid saponins as major chemical constituents of this plant (Ghosal et al., Citation1980). We have also reported that glucosylated furofurano lignans isolated from petroleum ether and ethanol extracts are responsible for the antistress activity exhibited by this plant. Justicisaponin-I, a triterpenoid saponin isolated from the ethanol extract of Justicia simplex., showed antispermicidal action (Ghosal et al., Citation1981). Lignans isolated from the Justicia. genus are well-known for their antitumor, antiplatelet, antiviral, and antidepressant activities (Rios et al., Citation2002). However, there exists no scientific investigation on the hepatoprotective effect of Justicia simplex.. In our continuing search to evaluate the medicinal uses of this plant, petroleum ether extract of Justicia simplex. whole plant and the isolated compounds were tested for protective action in CCl4-induced liver damage in albino rats. CCl4 toxicity is the model commonly used to study liver injury (Ko et al., Citation1993), and the intoxication induces necrosis and steatosis of the liver (Rubin et al., Citation1963). Liver is a vital organ involved in metabolism of foreign compounds entering the body. Human exposure to toxic substances and or intake of contaminated food causes liver toxicity. Conventional drugs used to cure liver toxicity are inefficient, and there is a need to find drugs that possess improved liver protection. The versatile nature of Justicia simplex. as a medicinal plant encouraged us to undertake the current study.
Materials and Methods
Plant material, extraction, and isolation
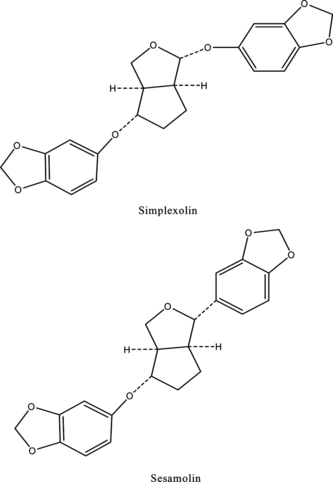
Justicia simplex. was collected from Ranikhet and was identified by the botanist from the Department of Botany, Banaras Hindu University, Varanasi, India. A voucher specimen has been preserved in the Pharmaceutical Chemistry Research Laboratory, Department of Pharmaceutics, Banaras Hindu University. The whole plant (2 kg) was dried, milled, and continuously extracted with petroleum ether (fraction 60–80°C) in a Soxhlet apparatus for 48 h. The extract was evaporated until the solvent was completely removed. The extract gave a yellow gummy material (56 g). This extract was processed in the same way as previously reported for the isolation of simplexolin and sesamolin (), and the compounds were identified by direct comparison with the authentic sample (mmp and co-TLC) and spectroscopic data (MS, IR, and 1H NMR) (Ghosal et al., Citation1979).
Animals
Male albino rats (Charles foster strain) weighing 100–150 g were obtained from the Central Animal House (reg. no. 542/02/ab/CPCSEA), Institute of Medical Sciences, Banaras Hindu University. Three rats per cage were kept and maintained on a 12-h light/dark cycle at an ambient temperature. The animals had free access to standard pellet diet (M/s Hindustan Lever Ltd, Mumbai, India) and water ad libitum..
Chemicals
Ellman's reagent was obtained from Sisco Laborotaries (Varanasi, India). Thiobarbituric acid (TBA) and 1,1, 3,3-tetraethoxypropane (TEP) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade. Span diagnostic kits were used for the spectrometric enzyme assays.
Experimental design
The experimental animals were divided into six groups of six animals each. Group I was marked as control. In group II (intoxicated), hepatotoxicity was induced by oral administration of CCl4 (2 mL/kg in 50% paraffin) 48 h before decapitation. Group III served as standard and was given the herbal preparation Nirocil (Solumicks Division, Bombay, India) at the dose of 100 mg/kg, p.o. Groups IV, V, and VI were given petroleum ether extract (JSE; 100 mg/kg, p.o.), simplexolin (10 mg/kg, p.o.), and sesamolin (10 mg/kg, p.o.) as 0.3% suspension in carboxyl methyl cellulose (CMC), respectively, for 6 consecutive days. The dose of the extract was ascertained by pilot studies over a range of dosages varying from 50 to 200 mg/kg, and also treatment was done with the isolated compounds over the dosage range 5–20 mg/kg. The plant extract and the isolated compounds did not show any toxicity over the tested dosage range. On day 7, animals were sacrificed by decapitation. The blood was collected by cardiac puncture and centrifuged to separate the serum. Serum was used for the estimation of ALT (alanine aminotranerase) (Mohur & Cook, Citation1957), AST (aspartate aminotransferase) (Mohur & Cook, Citation1957), ALP (alkaline phosphatase), LPO (lipid peroxidation) (Ohkawa et al., Citation1979), total protein (Lowry et al., Citation1951), and TG (triglycerides) using the spectrometric diagnostic kits.
The liver tissue was excised immediately and washed with ice-cold saline. A portion of liver tissue was homogenized with potassium chloride (1.15% w/v), and the homogenate was used for the estimation of lipid peroxidation (Ohkawa et al., Citation1979) and hepatic GSH (glutathione) (Sedlak & Lindsay, Citation1986).
Statistical analysis
Statistical analysis was done by Student's t.-test. The results were considered significant if p < 0.05.
Results
Hepatic toxicity induced by CCl4 treatment
The hepatic damage induced by CCl4 was significant as evidenced from significant (p < 0.001) increase in serum levels of ALT, AST, ALP, and LPO compared with normal rats as given in . The levels of total protein and TG were also decreased significantly (p < 0.001) in intoxicated rats than in the normal rats (). CCl4 toxicity significantly decreased (p < 0.001) the hepatic GSH level and showed an increase (p < 0.001) in LPO compared with the normal group ().
Table 1. Effect of the Justicia simplex. crude extract and isolated lignans on the biochemical parameters of CCl4-intoxicated rats (n = 6, mean ± SEM)
Table 2. Effect of Justicia simplex. crude extract and isolated lignans on glutathione depletion and lipid peroxidation in CCl4-intoxicated rats (n = 6, mean±SEM)
Protective effect of pretreatment with the lignans
Pretreatment of rats with JSE and isolated simplexolin and sesamolin decreased the levels of hepatic enzymes, serum and hepatic LPO and increased the levels of serum protein, TG, and hepatic GSH at a significant level (p < 0.001) indicating the protective effect of the lignans under study in the pretreated rats. (Tables and ).
Discussion
Administration of CCl4 caused hepatic damage due to the free radical formed from CCl4 by the activation of the NADPH-Cyt P450 system of liver endoplasmic reticulum (Reckangale et al., Citation1974). This led to functional and morphologic changes in the cell membrane and resulted in leakage of hepatic enzymes. When compared with other toxins, CCl4 is known to cause hepatic damage with a marked increase in blood serum transaminases and phosphatase activity (Tiegs & Wendel, Citation1988; Dwivedi et al., Citation1990). The oxidation of fatty acids by free trichloromethyl radical (
) liberates lipid peroxides (Reckangale & Ghosal, Citation1966). The current study also proves these effects of CCl4 toxicity by the marked increase in serum hepatic enzymes and LPO levels (). Impairment of hepatic antioxidant status by CCl4 was evident by the increase in tissue LPO and decrease in GSH levels. Depletion of available GSH resulted in enhanced LPO and decreased GST activity (Anandan et al., Citation1999) as shown in .
Pretreatment with JSE (100 mg/kg) and simplexolin (10 mg/kg) and sesamolin (10 mg/kg) significantly (p < 0.001) normalized these enzyme levels compared with the intoxicated rats (). The pretreated rats showed significant (p < 0.001) hepatoprotective effect to CCl4 toxicity by decreased tissue susceptibility to peroxide-induced toxicity as supported by decrease in tissue lipid peroxide and GSH levels (). It has been suggested that methylenedioxy group in certain lignans is a critical feature for antihepatotoxic activity and could act through inhibition of lipid peroxidation by a nonenzymatic step (Kiso et al., Citation1985). The lignans used in this study also contained methylenedioxy group, which could be responsible for the protective effect exerted by these lignans. Previous studies have revealed that furofuran lignans like sesamolinol and schisanhenol have exhibited antioxidant properties through their metabolites (Potterat, Citation1997). The in vivo. metabolites of lignans used in the current study might also be responsible for their hepatoprotection through their antioxidant potential. The exact mechanism and structural feature responsible for the hepatoprotection needs further investigation. There are many reports about hepatoprotective effect of lignans but this is the first report of hepatoprotection by an aryloxy furofuran lignan isolated from Justicia simplex..
Acknowledgment
We are thankful to UGC, New Delhi, India, for a Senior Research Fellowship awarded to Mrs. S. Jasemine.
References
- Anandan R, Rekha RD, Devaki T (1999): Protective effect of Picrorrhiza kurroa. on mitochondrial glutathione antioxidant system in D-galactossamine-induced hepatitis in rats. Curr Sci 76: 1543–1545.
- Dwivedi Y, Rastogi R, Chander R, Sharma SK, Kapoor NK, Garg NK, Dhawan BN (1990): Hepatoprotective activity of picroliv against carbon tetrachloride induced liver damage in rats. Ind J Med Res 92B: 195–200.
- Ghosal S, Banerjee S, Srivastava RS (1979): Simplexolin, a new lignan from Justicia simplex.. Phytochemistry 18: 503–505.
- Ghosal S, Srivastava AK, Srivastava RS, Chattopadhyay S, Maitra M (1981): Justicisaponin-1, a new triterpenoid saponin from Justicia simplex.. Planta Med 42: 280–283.
- Ghosal S, Banerjee S, Jaiswal DK (1980): New furofurano lignans from Justicia simplex.. Phytochemistry 19: 332–334.
- Kiso Y, Tohkin M, Hikino H, Ikeya Y, Taguchi H (1985): Mechanism of antitoxic activity of wuweizisu Cand gomisin A. Planta Med 51: 331–334.
- Ko KM, Yick KP, Chiu TW (1993): Impaired hepatic antioxidant status in carbon tetrachloride intoxicated rats an in vivo. model for screening herbal extracts with antioxidant activities. Fitoterapia 64: 539–544.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin-phenol reagent. J Biol Chem 193: 265–275.
- Mohur A, Cook IJY (1957): Simple methods for measuring serum levels of glutamic–oxalo acetic and glutamic-pyruvic transaminase in routine laboratories. J Clin Pathol 10: 394–399.
- Ohkawa H, Onishi N, Yagi K (1979): Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Potterat O (1997) Antioxidants and free radical scavengers of natural origin. Curr Org Chem 1: 415–440.
- Reckangale RO, Ghosal, AK (1966): Quantitative estimation of peroxidative degradation of rat liver microsomal and mitochondrial lipids after carbontetrachloride poisoning. Exp Mol Pathol 5: 413–426.
- Reckangale RO, Glende EA, Ugazio G, Koch RR, Srinivasan S (1974): New data in support of lipid peroxidation of carbon tetrachloride liver injury. Israeli J Med Sci 10: 301–307.
- Rios JL, Giner RM, Prieto JM (2002): New findings on the bioactivity of lignans. Studies Nat Prod 26(G): 183–292.
- Rubin E, Hutter F, Proper H (1963): Cell proliferation and fiber formation in chronic carbon tetrachloride intoxication, a morphological and chemical study. Am J Pathol 42: 715.
- Sedlak L, Lindsay L (1986): Estimation of protein bound and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192–205.
- Tiegs G, Wendel A (1988): Leukotriene-mediated liver injury. Biochem Pharmacol 37: 2569–2573.