Abstract
Bioassay-guided fractionation of the MeOH extracts of Euphorbia hirta. Linn (Euphorbiaceae) aerial parts led to the isolation of flavonol glycosides afzelin (1), quercitrin (2), and myricitrin (3), whose structures were established by MS and NMR analysis. Compounds 1–3 showed proliferation inhibition of Plasmodium falciparum., with IC50 values of 1.1, 4.1, 5.4 µg/mL, repectively. On the other hand, they exhibited little cytotoxic property against human epidermoid carcinoma KB 3-1 cells.
Introduction
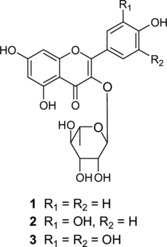
Malaria is one of the three killers among communicable diseases in the world today, infecting approximately 300–500 million people every year. Mortality exceeds 1–3 million people per year, most of them being children in Africa under the age of 4 years, a number that is continuously increasing due to the rapid spread of drug-resistant Plasmodium. parasites (Greenwood & Mutabingwa, Citation2002; Schwikkard & van Heeden, Citation2002; Wright, Citation2005). After an antimalarial screening of Congolese medicinal plants, we have observed that the MeOH extract of Euphorbia hirta. Linn (Euphorbiaceae) (Oliver-Bever, Citation1986; Iwu, Citation1993) demonstrated not only potent in vitro. antimalarial activity against P. falciparum. but also little cytotoxic property against human epidermoid carcinoma KB 3-1 cells. The antimalarial activity of E. hirta. extracts has been previously reported (Tona et al., Citation1999Citation2004; Köhler et al., Citation2002; Koli et al., Citation2002), in addition to other healing properties, such as antiulcer (Lin & Hsu, Citation1988), antimicrobial (Oyewale et al., Citation2002; Sudhakar et al., Citation2006), antibacterial (Satyanarayana & Singhai, Citation1979; Vijaya et al., Citation1995), anti-Helicobacter pylori. (Wang & Huang, Citation2005), sedative and anxiolytic (Lanhers et al., Citation1990), neurophysiologic (Lanhers et al., Citation1996), antihypertensive (Williams et al., Citation1997; Johnson et al., Citation1999), antihistaminic and immunosuppressive (Singh et al., Citation2006), molluscicidal (Singh et al., Citation2005), antiamebic (Tona et al., Citation2000), antifungal (Masood & Rajan, Citation1991), spasmolytic (Tona et al., Citation2000), antidiarrheic (Galvez et al., Citation1993aCitation1993b; Mallavadhani et al., Citation2002; Hore et al., Citation2006), analgesic and antipyretic (Lanhers et al., Citation1991), and anti-inflammatory (Lanhers et al., Citation1991; Martinez-Vazquez et al., Citation1999; Singh et al., Citation2006). Phytochemical investigation on this species led to the isolation of tannins (Lanhers et al., Citation1991; Yoshida et al., Citation1988Citation1990aCitation1990b), flavonoids (Lin & Hsu, Citation1988; Lanhers et al., Citation1991; Galvez et al., Citation1993aCitation1993b; Aquiland & Zhan, Citation1999; Koli et al., Citation2002; Oyewale et al., Citation2002; Tona et al., Citation2004), triterpenes (Martinez-Vazquez et al., Citation1999), phenolic acids, saponins, and amino acids (Lanhers et al., Citation1991). In this communication, we report the bioassay-guided isolation of the antimalarial principles of E. hirta. MeOH extract, which were identified by spectroscopic methods as afzelin (1), quercitrin (2), and myricitrin (3) () (Zhang et al., Citation2003).
Materials and Methods
General experimental procedures
1H and 13C NMR spectra were recorded on a JNM-GX-500 (JEOL, Tokyo, Japan) spectrometer. Chemical shifts were reported with reference to the respective residual solvent peaks (δH 3.30 and δC 49.0 for CD3OD). Fast Atom Bombardment Mass Spectometry (FABMS) data were obtained on a JMS SX-102 (JEOL) instrument using m.-nitrobenzyl alcohol as the matrix. For flash column chromatography, silica gel (BW-200, 400-500 mesh, Fuji Sylisia) was used, whereas thin-layer chromatography (TLC) and high performance thin layer chromatography (HPTLC) analyses were carried out over precoated plates (Merck, Kiesel gel 60F254, 0.25 mm, and RP-18 WF254, respectively). Spots were visualized under UV 254 and 366 nm, and 1% Ce(SO4)2/10% H2SO4, p.-anisaldehyde/H2SO4 (AcOH 5 mL, c.-H2SO4 25 mL, EtOH 425 mL, water 25 mL) spray reagents. Reversed-phase HPLC was performed on a semipreparative Cosmosil C18-AR-II column (250 × 10 mm, 4 µm 80 Å), using a Shimadzu SPD-10A vp UV-Vis detector.
Plant material
Euphorbia hirta. was collected in Kinshasa, Congo, in July 2001. A voucher specimen was deposited in the Medicinal Plants Source Exploration Lab, Graduate School of Pharmaceutical Science, Osaka University, Japan.
Extraction
The air-dried aerial parts of Euphorbia hirta. (300 g) were cut into small pieces and successively extracted one-time with MeOH at room temperature and three-times at 75°C under reflux. The extracts were combined and concentrated in vacuum to obtain a residue that showed growth inhibition of P. falciparum.. A part of this extract (3 g) was partitioned between water and EtOAc to yield an active organic fraction (1 g) that was then subjected to normal-phase flash chromatography (hexane:EtOAc, 10:1, hexane:EtOAc, 3:1, and MeOH elution) to afford four fractions (E1–E4). Fraction E-4 (647 mg) showed 90% growth inhibition of P. falciparum. at a concentration of 5 µg/mL and little cytotoxicity against KB3-1 cells up to a concentration of 50 µg/mL. Flash chromatography of this fraction, eluted with CHCl3:MeOH:H2O, 30:3:1 (lower layer), CHCl3:MeOH:H2O, 10:3:1 (lower layer), and MeOH, afforded five fractions (E41–E45), which were monitored againstP. falciparum. and KB3-1 cells. The active fraction (E44, 204 mg) was submitted to RP-18 HPLC (MeCN:H2O, 25:75) to yield three fractions (E443, E442, and E444), containing compounds 1 (2.9 mg), 2 (6.5 mg), and 3 (2.7 mg), respectively, which were further purified by HPLC.
In vitro. antimalarial bioassays
Quinine (Nacalai, Tokyo, Japan) was used as a positive control for in vitro. antimalarial experiments. The stock solutions of drugs were prepared in DMSO and diluted with complete medium. DMSO concentration in culture medium never exceeded 1%, unless otherwise noted. Colchicine (Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) was used as a positive control in cytotoxic assay. Bioassays were carried out in a PVC clean bench (Hitachi, Tokyo, Japan). Parasites were incubated in a low-temperature 5% O2 and 5% CO2 controlled incubator (Model-9200, Wakenyaku, Co. Ltd., Kyoto, Japan), and the microscopic inspection of malaria parasites was done over under oil emersion (Olympus BX51, 100 × 1.25, Tokyo, Japan). Two strains of P. falciparum. (CDC1, chloroquine-sensitive; and FCR-3, cycloguanil-resistant from Gambia) were maintained in semiautomated continuous culture in medium RPMI 1640 containing HEPES buffer, heat-inactivated human serum (10%v/v), and gentamicin and cultured by standard methods.The parasites were synchronized at ring stage by sorbitol treatment. Initial parasitemia was adjusted to 0.5% with 2% hematocrit in all experiments (Krishna & Ganapaty, Citation1983). Growth inhibition, intraerythrocytic development, and parasite morphology were evaluated in culture by microscopic observation of Giemsa-stained thin blood films. Drug-free cultures were always used as controls. To evaluate growth inhibition, parasitemia was measured by counting 10,000 erythrocytes and is reported as the percentage of parasitized erythrocytes. Intraerythrocytic development was monitored by examining a minimum of 1000 parasitized cells on each film, for differential counting of rings, trophozoites, and schizonts. The proportion of each group was calculated as a percentage of the total parasitized cells. Repetitive dosing effect was evaluated against FCR-3 strain following a model proposed in literature (Bwijo et al., Citation1997). Parasites were exposed to daily dosing of drugs by replacing the culture medium with the same volume of fresh medium containing the drug for 6 consecutive days. The parasites treated with drug-free growth medium containing 0.1% DMSO was considered as control. Thin smears were prepared every 24 h for the assessment of parasitemia and parasite replication.
Evaluation of cytotoxicities
Human epidermoid carcinoma KB cells were cultured in RPMI 1640 medium with 0.58 mg/mL glutamine, 50 µg/mL kanamycin sulfate, supplemented with 10% fetal bovine serum, and incubated in 5% CO2 controlled incubator (Sensor; Sanyo, Nakashina, Japan) at 37°C. Cytotoxic activity was measured by means of MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) formazan colorimetric assay performed in 96-well plates (Nunclon, Roskilde, Denmark). Equal numbers of cells were inoculated into each well with 100 ▪L of the culture medium, and then a 100 µL solution of each tested compound was added to each well. After 72 h incubation under 5% CO2 atmosphere at 37°C, 25 µL of MTT solution (2 mg/mL in PBS) was added to each well and incubated for further 3 h. The percentage of cell growth inhibition was calculated from the absorbance at 540 nm, recorded on a microplate reader (BIO RAD 450, Hercules, CA, USA) (Krishna & Ganapaty, Citation1983). Inhibitory concentration 50% (IC50) values were determined by linear interpolation from the inhibition curve.
Results and Discussion
Bioassay-guided fractionation of the MeOH extract of E. hirta. aerial parts, monitored against P. falciparum. parasites, yielded a main active chromatographic fraction showing 90% growth inhibition of P. falciparum. at a concentration of 5 µg/mL and, in addition, little cytotoxic property against human epidermoid carcinoma KB 3-1 cells up to a concentration of 50 µg/mL. The active constituents were isolated by flash chromatography and semipreparative reversed-phase HPLC and identified as the flavonol glycosides afzelin (1), quercitrin (2), and myricitrin (3). Their structures were established by HRFABMS and NMR, whose data corresponded with that published in literature (Zhang et al., Citation2003). Compounds 1–3 inhibited the proliferation of P. falciparum. (CDC1); IC50 values 1.1, 4.1, 5.4 µg/mL, repectively. On the other hand, these flavonol glycosides have little influence on the growth of KB 3-1 representing the host cell, showing cytotoxic activity; IC50 values 276.1, 88.2, 156.4 µg/mL, respectively. This effect was previously demonstrated for quercitrin and other naturally occurring flavonol glycosides isolated from a Japanese traditional crude drug (Murakami et al., Citation2001). Quercitrin is also a recognized antidiarrheic agent of E. hirta. (Galvez et al., Citation1993b; Mallavadhani et al., Citation2002; Hore et al., Citation2006). Although afzelin and myricetin have been previously isolated from an E. hirta. specimen collected in Taiwan (Lin & Hsu, Citation1988), their characterization as antimalarial principles is here reported for the first time.
On the other hand, the CDC1 and FCR-3 strains showed similar IC50 results. The effects of flavonol glycosides on the life cycle of P. falciparum. were investigated. It was shown that monoglycoside 2 arrested the life cycle of the parasite irreversibly at the trophozoite stage. P. falciparum. possesses a 48-h intraerythrocytic growth cycle, which is morphologically defined as ring, trophozoite, and schizont in a chronological order. A mature parasite can produce around 20 merozoites, with each merozoite able to invade other erythrocytes (Miller et al., Citation2002). As a result, the infection ratio increases many-fold after a single cycle. This cyclical way of invasion to new erythrocytes progresses until the death of the host. The effect of flavonol glycosides on the life cycle of malaria parasites was examined against the synchronized culture of ring stage parasite with inoculation of samples at 0 h. It was shown that flavonol monoglycoside 2 irreversibly arrested the life cycle of the parasites beyond the transition from trophozoite stage (24 h) to schizont stage (28–32 h). However, 2 was shown to kill the parasite dose-dependently between 0.5 and 5 µg/mL and exhibited complete growth inhibition of P. falciparum. at a dose of 10 µg/mL.
Acknowledgments
This study was supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan; the K. C. Wong Education Foundation, Hong Kong; and by a grant from the Hundred Talents Project of the Chinese Academy of Sciences.
References
- Aquiland M, Khan IZ (1999): Euphorbianin—a new flavonol glycoside from Euphorbia hirta. Linn. Global J Pure Appl Sci 5: 371–373.
- Bwijo B, Alin MH, Abbas N, Ericsson O, Björkman A (1997): Repetitive dosing of artemisinin and quinine against Plasmodium falciparum in vitro.: A simulation of the in vivo. pharmacokinetics. Acta Tropica 65: 11–22.
- Galvez J, Zarzuelo A, Crespo ME (1993a): Antidiarrhoeic activity of Euphorbia hirta. extract and isolation of active flavonoid constituent. Planta Med 59: 333–336.
- Galvez J, Crespo ME, Jimenez J (1993b): Antidiarrhoeic activity of quercitrin in mice and rats. J Pharm Pharmacol 45: 157–159.
- Greenwood B, Mutabingwa T (2002): Malaria in 2002. Nature 415: 670–673.
- Hore SK, Ahuja V, Mehta G (2006): Effect of aqueous Euphorbia hirta. leaf extract on gastrointestinal motility. Fitoterapia 77: 35–38.
- Iwu MM (1993): Handbook of African Medicinal Plants.. Boca Raton, CRC Press, pp. 26–28.
- Johnson PB, Abdurahman EM, Tiam EA (1999): Euphorbia hirta. leaf extracts increase urine output and electrolytes in rats. J Ethnopharmacol 65: 63–69.
- Köhler I, Siems KJ, Kfarft C (2002): Herbal remedies traditionally used against malaria in Ghana: Bioassay-guided fractionation of Microglassa pyrifolia. (Asteraceae). Z Naturforsch 57C: 1022–1027.
- Koli MC, Choudhary R, Kumar S (2002): An isoflavone glycoside from the stem of Euphorbia hirta. Linn as antimalarial compound. Asian J Chem 14: 1673–1677.
- Krishna RCV, Ganapaty S (1983): Investigation on Euphorbia pilulifera.. L Fitoterapia 54: 265–267.
- Lanhers MC, Fleurentin J, Cabalion P (1990): Behavioral effects of Euphorbia hirta. L.: Sedative and anxiolytic properties. J Ethnopharmacol 29: 189–198.
- Lanhers MC, Fleurentin J, Dorfman P(1991): Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta.. Planta Med 57: 225–231.
- Lanhers MC, Fleurentin J, Dorfman P (1996): Neurophysiological effects of Euphorbia hirta. L. (Euphorbiaceae). Phytother Res 10: 670–676.
- Lin Y-L, Hsu S-Y (1988): The constituents of the antiulcer fractions of Euphorbia hirta.. Chin Pharm J 40: 49–51.
- Mallavadhani UV, Sahu G, Muralidhar J (2002): Quantitative estimation of an antidiarrhoeic marker in Euphorbia hirta. samples. Pharm Biol 40: 103–106.
- Martinez-Vazquez MM, Apan TOR, Lazcano ME, Bye R (1999): Anti-inflammatory active compounds from the n.-hexane extract of Euphorbia hirta.. Rev Soc Quim Mex 43: 103–105.
- Masood A, Rajan KS (1991): The effect of aqueous plant extracts on growth and aflatoxin production by Aspergillus flavus.. Lett Appl Microbiol 13: 32–34.
- Miller HL, Baruch DI, Marsh K, Doumbo OK (2002): Parasite life cycle and pathogenesis of falciparum malaria. Nature 415: 673–679.
- Murakami N, Mostaqul HM, Tamura S, Itagaki S, Horii T, Kobayashi M (2001): New anti-malarial flavonol glycoside from Hydrangea dulcis. folium. Bioorg Med Chem Lett 11: 2445–2447.
- Oliver-Bever B (1986): Medicinal Plants in Tropical West Africa. London, Cambridge University Press, pp. 469–473.
- Oyewale AO, Mika A, Peters FA (2002): Phytochemical, cytotoxicity and microbial screening of Euphorbia hirta.. global J Pure Appl Sci 8: 49–55.
- Satyanarayana T, Singhai M (1979): Studies on antibacterial activity of Euphorbia hirta.. Indian Drugs Pharm Ind 14: 27–28.
- Schwikkard S, van Heeden FR (2002): Antimalarial activity of plant metabolites. Nat Prod Rep 19: 675–692.
- Singh GD, Kaiser P, Youssouf MS, Singh S, Khajuria A, Koul A, Bani S, Kapahi BK, Satti NK, Suri KA, John RK (2006): Inhibition of early and late phase allergic reactions by Euphorbia hirta. L. Phytother Res 20: 316–321.
- Singh SK, Yadav RP, Tiwari S, Singh A (2005): Toxic effect of stem bark and leaf of Euphorbia hirta. plant against freshwater vector snail Lymnaea acuminate.. Chemosphere 59: 263–270.
- Sudhakar M, Rao CV, Rao PM, Raju DB, Venkateswarlu Y (2006): Antimicrobial activity of Caesalpinia pulcherrima., Euphorbia hirta. and Asystasia gangeticum.. Fitoterapia 77: 378–380.
- Tona L, Ngimbi NP, Tsakala M, Mesia K, Cimanga K, Apers S, De Bruyne T, Pieters L, Totté J, Vlietinck AJ (1999): Antimalarial activity of 20 crude extracts from nine African medicinal plants in Kinshasa, Congo. J Ethnopharmacol 68: 193–203.
- Tona L, Kambu K, Ngimbi N, Mesia K, Penge O, Lusakibanza M, Cimanga K, De Bruyne T, Apers S, Totté J, Pieters L, Vlietinck AJ (2000): Antiamoebic and spasmolytic activities of extracts from some antidiarrhoeal traditional preparations used in Kinshasa, Congo. Phytomedicine 7: 31–38.
- Tona L, Cimanga RK, Mesia GK, Musuamba CT, de Bruyne T, Apers S, Hermans N, van Miert S, Pieters L, Totté J, Vlietinck AJ (2004): In vitro. antiplasmodial activity of extracts and fractions from seven medicinal plants used in Democratic Republic of Congo. J Ethnopharmacol 93: 27–32.
- Vijaya K, Ananthan S, Nalini R (1995): Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis.) and Euphorbia hirta. on Shigella. spp.—a cell culture study. J Ethnopharmacol 49: 115–118.
- Wang YC, Huang TL (2005): Screening of anti-Helicobacter pylori. herbs deriving from Taiwanese folk medicinal plants. FEMS Immunol Med Microbiol 43: 295–300.
- Williams LAD, Williams MG, Sajabi A, Barton EN, Fleischhacker R (1997): Angiotensin converting enzyme inhibiting and anti-dipsogenic activities of Euphorbia hirta. extracts. Phytother Res 11: 401–402.
- Wright CW (2005): Plant derived antimalarial agents: New leads and challenges. Phytochem Rev 4: 55–61.
- Yoshida T, Chen L, Shingu T, Okuda T (1988): Tannins and related polyphenols of Euphorbiaceaous plants. IV. Euphorbins A and B, novel dimeric dehydroellagitannins from Euphorbia hirta. L. Chem Pharm Bull 36: 2940–2949.
- Yoshida T, Namba O, Chen L, Okuda T (1990a): Tannis and related polyphenols of Euphorbiaceae plants. V. Euphorbin C, an equilibrated dimeric dehydroellagitannin having a new tetrameic galloyl group. Chem Pharm Bull 38: 86–93.
- Yoshida T, Namba O, chen L, Okuda T (1990b): Euphorbin C, a hydrolysable tannin dimmer of highly oxidized structure, from Euphorbia hirta.. Chem Pharm Bull 38: 1113–1115.
- Zhang Z, ELSohly HN, Li X-C, Khan SI, Khan SI, Broedel SE, Jr, Raulli RE, Ciglar RL, Burandt C, Walker LA (2003): Phenolic compounds from Nymphae odorata.. J Nat Prod 66: 548–550.