Abstract
The ethyl acetate extract of Eclipta prostrata. L. (Asteraceae) was evaluated for its antivenom potential against Calloselasma rhodostoma. Kuhl (Viperidae) (Malayan pit viper; MPV) venom. The partially purified ethyl acetate extract (PEE) was found to contain 47% wedelolactone as its major constituent. PEE and wedelolactone demonstrated strong antiproteolytic and antihemorrhagic activities against MPV venom in a dose-dependent manner. The extract, at 5 mg/mL, could inhibit proteolytic activity of 100 µg of the venom and hemorrhagic activity of 3 minimum hemorrhagic doses (MHD) to 95% and 68%, respectively. At the same concentration, wedelolactone could neutralize the proteolytic activity at around 76% and, at doses of 0.25–1 mg/mL, offered protection against hemorrhagic activity of the venom in the range 3–35%. Both PEE and wedelolactone displayed partial anti–phospholipase A2 activity (21% for PEE and 7% for wedelolactone) and could not neutralize the lethal effect of either 2LD50 or 4LD50 of MPV venom.
Introduction
Malayan pit viper (Calloselasma rhodostoma. Kuhl [Viperidae]) is one of the most common venomous snakes in local areas of Thailand, posing an occupational hazard to both rubber plantation workers and farmers (Viravan et al., Citation1992; Chanhome et al., Citation1998). Several enzymes and toxins in Malayan pit viper (MPV) venom can cause many pharmacological responses in the snakebite victims (Ouyang et al., Citation1983; Tan et al., Citation1986; Ponnudurai et al., Citation1993). This snake venom mainly acts on the hemostatic system, particularly on blood clotting cascade (Reid & Chan, Citation1968; Devaraj, Citation1979; Bjarnason & Fox, Citation1994; Dambisya et al., Citation1994; Markland, Citation1998). The victim may die from severe effects of the venom itself or other contributing factors such as intracranial hemorrhage, shock, and inappropriate or inadequate dose of the antivenin (Looareesuwan et al., Citation1988).
We have recently reported in vitro. studies of the inhibitory activities of some medicinal plants against snake venom and suggested the benefits of using medicinal plants as first aid in the treatment of local tissue damage or necrosis where serotherapy is not effective (Pithayanukul et al., Citation2004Citation2005). Eclipta prostrata. L. (Asteraceae) is one of the herbaceous plants reputed to antagonize snake venom in both China and Brazil (Mors, Citation1991). This weed plant grows abundantly in cool, moist areas of tropical and subtropical regions. There have been several reports about its constituents (Gondachari et al., Citation1956; Krishnaswamy et al., Citation1966; Bhargava et al., Citation1972), and a number of biological effects could be attributed to the extracts of this plant (Khin et al., Citation1978; Wagner & Fessler, Citation1986; Leal et al., Citation2000). In addition, the extracts and their constituents (wedelolactone, sitosterol, and stigmasterol) have been demonstrated as effective in neutralizing lethal, myotoxic, hemorrhagic, proteolytic, and phospholipase A2 activities of some crotalinae snake venoms or their toxins in animal model (Mors et al., Citation1989; Melo et al., Citation1994Citation1997; Melo & Ownby, Citation1999). Butanol extract of Eclipta prostrata., containing high concentration of demethylwedelolactone, demonstrated high potential in neutralizing lethal and hemorrhagic activities of MPV venom (Pithayanukul et al., Citation2004). However, there has been no report on the enzyme neutralizing and inhibitory activities of wedelolactone and other constituents of this plant against this snake venom. Thus, the objectives of this study were to determine the potential of wedelolactone and the ethyl acetate extract of Eclipta prostrata. in alleviating the venom activities of Malayan pit viper.
Materials and Methods
Plant material
The aerial part of Eclipta prostrata. was collected from Nonthaburi, Thailand, and identified by R. Bavovada. A voucher specimen (no. P.B. 20005) has been deposited at the Museum of Natural Medicine, Faculty of Pharmaceutical Sciences, Chulalongkorn University (Bangkok Thailand).
Plant extraction and identification of major constituent
The plant was extracted following the method of Wagner and Fessler (Citation1986) with slight modification. Fresh aerial part of Eclipta prostrata. was extracted with methanol at 60–80°C in a Soxhlet apparatus for 48 h. The solvent was removed under reduced pressure to yield a viscous mass that was further suspended in water and heated at 80°C. The aqueous suspension was centrifuged at 2000 rpm for 10 min, and then the aqueous supernatant was partitioned with ethyl acetate. After the solvent was removed, the ethyl acetate extract was subjected to a silica gel (E. Merck) quick column (6.5 cm i.d. × 4 cm, 30 g), using chloroform/MeOH = 5:1 as the mobile phase. The collected fractions were checked for the presence of wedelolactone, which appeared on TLC as bright blue spot under UV light and formed greenish color upon reaction with 5% ferric chloride test solution. The fractions containing wedelolactone were divided into two portions: one was evaporated to obtain dried brownish-green powder of partially purified ethyl acetate extract (PEE), and the other was further purified over a Sephadex LH-20 column using MeOH as eluting solvent to yield wedelolactone. The compound was identified by comparison of its mass spectrum and NMR spectral data with literature (Wagner et al., Citation1986).
Standardization
Wedelolactone content of PEE was determined using TLC densitometric scanning at the wavelength of 425 nm. Standard solutions of wedelolactone and PEE in MeOH were prepared at the concentrations of 0.37 and 1.17 mg/mL, respectively. The samples were applied on TLC plates using hexane/ethyl acetate = 2:3 as developing solvent. The plates were then sprayed with silver nitrate reagent and detected with TLC scanner.
Animals and venom
Swiss albino mice of both sexes weighing about 18–20 g and about 25–30 g were used for the lethal effect and hemorrhagic activity assays, respectively. The experiments were performed according to the international and Mahidol University's guidelines on animal studies. Lyophilized Malayan pit viper (MPV) venom was provided by Queen Saovabha Memorial Institute, Thai Red Cross Society, Thailand.
Anti–snake venom activity
Neutralization of lethality
The median lethal dose (LD50) of MPV venom was determined according to the method developed by Theakston and Reid (Citation1983). The venom in 0.2 mL of physiologic saline was injected into the tail vein of mice. Duplicated experiment was carried out in five mice at each venom dose. The LD50 was calculated with the confidence limit at 95% probability by the analysis of deaths occurring within 24 h of venom injection (Reed & Muench, Citation1938). The antilethal potentials of PEE and wedelolactone were determined against both 2LD50 and 4LD50 of MPV venom in three mice for each test concentration of the extract and pure compound. PEE was dissolved in a mixture of EtOH/PEG 400/water (4:1:5), while, for wedelolactone, DMSO was added in order to help dissolve the compound. The test solution was preincubated with the venom at 37°C for 1 h and centrifuged at 10,000 × g. for 10 min before 0.2 mL of the supernatant was injected through the tail vein of mice. Each experiment was done in triplicate. The solvents, the extract or wedelolactone solution, and the venom alone were used as controls.
Antihemorrhagic activity
Hemorrhagic activity was determined according to the method of Kondo et al. (Citation1960) and modified methods of Gutierrez et al. (Citation1985) and Otero et al. (Citation2000). The minimum hemorrhagic dose (MHD) of MPV venom was established by injecting 0.04 mL of different amount of venom (2–32 µg in physiologic saline) intradermally into two separately marked positions on the shaved dorsal skin of unanesthesized mice. After 1.5 h, mice were sacrificed with an overdose of chloroform, the dorsal skin was removed, and the diameter of the hemorrhagic lesion on the inner surface of the skin was measured using calipers and background illumination. The mean diameter of the hemorrhagic lesion was calculated for each venom dose. Dose-response curve between the venom dose and the diameter of the hemorrhagic lesion was plotted, and MHD was recorded as the dose that caused lesion diameter of 10 mm. The experiment was done in triplicate. The PEE (1.25–5 mg/mL) test solutions were prepared in EtOH/PEG 400/water (1:2:7), whereas those of wedelolactone (0.3–1 mg/mL) were prepared in similar solvent mixture with DMSO added to completely dissolve the compound. Antihemorrhagic potential of the test solutions was determined against 3MHD of the venom. Four mice were used for each tested dose, and the experiments were done in triplicate. Equal volumes (0.1 mL each) of the test solution and MPV venom solution were mixed, then incubated at 37°C for 1 h and centrifuged at 10,000 × g. for 10 min. An aliquot (0.04 mL) of the supernatant containing 3MHD of the venom was then injected intradermally at marked positions on the shaved dorsal skin of unanesthesized mice. After the mice were sacrificed, the diameters of the hemorrhagic lesions were measured. The antihemorrhagic activities of wedelolactone and PEE were calculated as percent inhibition. The venom and the test solutions were used as controls.
Antiproteolytic activity
Modified methods of Kunitz (Citation1947) and Tan et al. (Citation1986) were employed in measuring the proteolytic activity of MPV. Two milliliters of 1% casein in 0.25 M sodium phosphate buffer pH 7.75 and 0.1 mL of venom (10–2000 µg) in physiologic saline were incubated for 1 h at 37°C. The undigested casein was precipitated and the reaction terminated by adding 2 mL of 5% trichloroacetic acid. After centrifugation at 10,000 × g. for 10 min, the absorbance of the supernatant was measured at 280 nm. One unit of proteolytic activity was defined as an increase of 0.001 absorbance units at 280 nm per hour. The initial proteolytic dose of MPV venom was obtained from the plot between proteolytic activity and the venom doses. Solutions of 0.5–5 mg/mL PEE and wedelolactone were evaluated for their antiproteolytic potentials against MPV venom. Each 0.05 mL of the test solution was preincubated at 37°C with an equal amount of the venom solution (1 mg/mL) for 1 h before the mixture was subjected to proteolytic activity evaluation. The experiments were performed three times.
Anti–phospholipase A2 activity
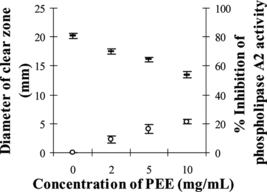
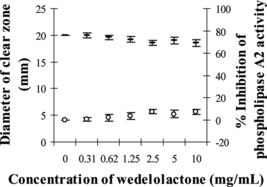
Phospholipase A2 activity was measured using an indirect hemolytic assay on agarose–erythrocyte–egg yolk gel plate to define the minimum indirect hemolytic dose (Gutierrez et al., Citation1988; Otero et al., Citation2000). The minimum indirect hemolytic dose (MIHD) of MPV venom (1.8 µg) was the dose that, after incubation for 20 h at 37°C, induced hemolysis halo having the diameter of 20 mm. Both PEE (at 2–10 mg/mL) and wedelolactone (at 0.3–10 mg/mL) were tested against 1 MIHD of the venom. Test solutions and MPV venom, 0.05 mL each, were preincubated for 1 h at 37°C. After centrifugation at 10,000 × g. for 10 min, the supernatant was tested for phospholipase A2 activity. The anti–phospholipase A2 potential of the extract and wedelolactone was expressed as percent inhibition of the enzyme activity, in which 100% inhibition should produce no clear zone. The experiments were performed three times.
Gel electrophoresis (SDS-PAGE) of venom proteins
After the inhibition of lethality test, venom proteins in the supernatants and in the precipitates of the venom-extract or venom-wedelolactone mixtures were analyzed by gel electrophoresis (SDS-PAGE) and compared with standard molecular weight markers including bovine serum albumin, ovalbumin, soybean trypsin inhibitor, lysozyme, and cobra neurotoxin. The method of Laemmli (Citation1970) was followed with a slight modification. The results were confirmed by using gel scanner to measure the optical densities of the proteins at 280 nm.
Statistical analysis
The survival rate and the inhibition of hemorrhagic activity in mice that received mixtures of venom and ethyl acetate extract or wedelolactone were compared at different time intervals with the controls (receiving the venom, extract, or compound alone). Analysis of variance (ANOVA) was used to determine the significance (p < 0.05) of the data obtained in all experiments.
Results
Wedelolactone content of the extract
Monitoring TLC chromatograms under UV light at 254 nm indicated that, in addition to wedelolactone, which was the major component, there were many minor constituents in the ethyl acetate extract of Eclipta prostrata.. The yields of ethyl acetate fraction and PEE from the whole plant were 0.34% and 0.011%, respectively. The content of wedelolactone in PEE was 47%.
Neutralization of lethality
The LD50 of MPV venom was determined as 114.77±11.70 µg/mouse. It was found that PEE (0.6–10 mg/mouse) and wedelolactone (0.75–10 mg/mouse) could not neutralize either 2LD50 or 4LD50 of MPV venom. The animals showed typical toxic signs of tremor, tachypnea, dyspnea, alteration in consciousness and local reaction effect or rapid edema with ecchymosis at the injection site and died within 1 h after receiving MPV venom. The solutions of PEE, wedelolactone, and the solvents used alone produced neither lethal effect nor typical toxic signs at the tested doses.
Inhibition of hemorrhagic activity
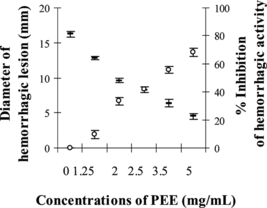
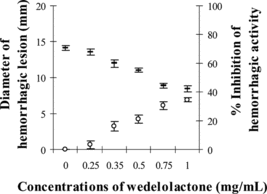
The minimum hemorrhagic dose (MHD) of MPV venom was determined as 12.94 µg, and at 3MHD the venom induced hemorrhagic lesion with the diameter of 16.26±0.44 mm. showed the reduction in diameter of hemorrhagic lesion to 12.82–4.50 mm when 1.25–5 mg/mL of PEE was preincubated with the venom. The protection against hemorrhagic activity of MPV venom by partially purified ethyl acetate extract of Eclipta prostrata. was calculated as up to 68% (at p < 0.05 compared with 3MHD plus solvent as the control). The pure compound, wedelolacton (), was able to partially reduce the diameter of hemorrhagic lesion induced by 3MHD of MPV venom (14.13±0.22 mm) to 13.54–8.90 mm at doses between 0.25 and 1 mg/mL. The calculated percent inhibition of hemorrhagic activity by wedelolactone was between 3% and 35%. Both the compound and extract at the tested doses, or the solvent used, did not induce hemorrhagic lesions to animal skin.
Antiproteolytic activity
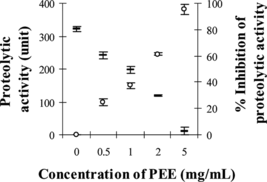
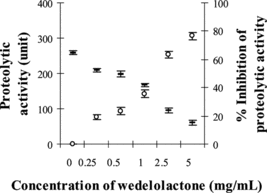
demonstrated that PEE (0.5–5 mg/mL) was able to significantly neutralize the proteolytic activity of the MPV venom at its initial proteolytic dose of 100 µg to between 241 and 11 units (p < 0.05, compared with venom plus solvent as the control). This indicates protection by PEE against proteolytic activity of MPV venom of up to 95% in a dose-dependent manner. Wedelolactone (0.25–5 mg/mL) could also significantly neutralize this activity of MPV venom to between 209 and 61 units (p < 0.05). The protection by wedelolactone against proteolytic activity was up to 76%, as shown in .
Anti–phospholipase A2 activity
MPV venom at the amount of 1.6 µg, which produced clear zone diameter of 20.20±0.43 mm on agarose–erythrocyte–egg yolk gel plate, was selected as the minimum indirected hemolytic dose (MIHD). Partial inhibition of phospholipase A2 activity by PEE was observed as the diameters of clear zone were significantly decreased to 17.43–13.47 mm (p < 0.05) when 2–10 mg/mL of PEE were used (). The percent inhibition was 9–21%. However, wedelolactone at the concentrations between 0.3 and 10 mg/mL could only slightly inhibit 1 MIHD of MPV venom between 0.1% and 7% (p > 0.05; ). The solvent systems used had no effect on this study.
Analysis of venom proteins
The SDS-PAGE electrophoretograms demonstrated that when PEE and wedelolactone contents of the venom-extract or venom-compound mixtures were increased from 0.6 to 10 mg/mouse, the venom proteins in the supernatant gradually decreased in a dose-dependent manner and could be detected in the precipitates. This indicates slight precipitation of the venom proteins from the venom-PEE and venom-wedelolactone mixtures. The electrophoretogram results corresponded with optical densities of the proteins in the supernatant and the precipitates.
Discussion
Although the yields of PEE and wedelolactone from Eclipta prostrata. were rather low, their antagonistic potential against hemorrhagic and proteolytic activities of Malayan pit viper venom could be considered significantly strong, as shown in –4. This could be due to the neutralization of hemorrhagic toxins of MPV venom by both PEE and wedelolactone. Because the hemorrhagic activity of snake venoms has been ascribed to the presence of hemorrhagic metalloproteinases (Kini & Evans, Citation1992) that presumably act by destroying the collagenous basement membrane and other connective tissue collagen with consequent weakening of the stability of the blood vessel causing hemorrhagic effect (Ohsaka, Citation1979; Bjarnason & Fox, Citation1989), the major hemorrhagin of MPV venom, termed rhodostoxin. (Ponnudurai et al., Citation1993), could probably be neutralized by PEE and wedelolactone. Possible mechanism of enzyme inhibition might involve the complexation or chelation of the zinc atom required for catalytic activity of the venom's hemorrhagic metalloproteinases. Both PEE and wedelolactone should bind close to the enzyme's catalytic zinc, causing direct blockage. The results obtained here support other previous studies showing that when the zinc atom of hemorrhagic metalloproteinases was removed or chelated, their proteolytic and hemorrhagic activities were inhibited (Bjarnason & Tu, Citation1978; Tan et al., Citation1997; Castro et al., Citation1999; Bode & Huber, Citation2000; Valente et al., Citation2001). PEE at 5 mg/mL, equivalent to wedelolactone 2.35 mg/mL, could almost completely (95% inhibition) protect against proteolytic activity of MPV venom, whereas wedelolactone itself at 5 mg/mL could inhibit this venom activity to a lesser extent of 76%. This result directly indicates that other constituents of PEE apart from wedelolactone also imparted antiproteolytic activity and might work synergistically with this major compound in neutralizing the proteolytic action of MPV venom. In addition, although the inhibitory potential of PEE and wedelolactone against phospholipase A2 activity of the venom was low (up to 21% for PEE and 7% for wedelolactone at the same concentration of 10 mg/mL), the result confirms that there were other active constituents in PEE other than wedelolactone that might possess similar activities against MPV venom.
As for the antihemorrhagic activity, it appears that wedelolactone was mainly responsible for this potential of Eclipta prostrata. ethyl acetate extract, as wedelolactone at 1 mg/mL could diminish hemorrhagic diameter induced by the venom down to about 35%, whereas 5 mg/mL of PEE could cause the reduction of the diameter only to 68%.
Both PEE and wedelolactone could not neutralize the lethal effect of MPV venom in the in vitro. study either against 2LD50 or 4LD50 at all tested doses between 0.6 to 10 mg/mouse. This could be due to the limited solubility of both extracts in physiologic saline and various nontoxic polar solvent systems, thus limiting the tested doses to not more than 10 mg/mouse. The electrophoretogram and optical density results confirmed that although various proteins of the control MPV venom gradually decreased when mixed with increasing doses of PEE and wedelolactone, most of them still remained. Therefore, the amount of PEE and wedelolactone used in this assay might be inadequate to neutralize the lethality of MPV venom.
In conclusion, the ethyl acetate extract of Eclipta prostrata., which was composed mainly of the active wedelolactone and other constituents, displayed high potential in neutralizing both the proteolytic and hemorrhagic activities of MPV venom. The benefit of the extract in treating necrosis and inflammation from viper bites is therefore promising, especially in the prevention of local tissue necrosis, which normally cannot be treated by the administration of horse serum. Furthermore, effective inhibition of the venom enzymatic activities not only can reduce the local tissue damage but also can retard the diffusion of systemic toxins and thus increase survival rate of the victim of viper bite.
References
- Bhargava KK, Krishnaswamy NR, Seshadri TR (1972): Demethylwedelolactone glucoside from Eclipta alba. leaves. Indian J Chem 10: 810–811.
- Bjarnason JB, Fox JW (1989): Hemorrhagic toxins from snake venoms. J Toxicol Toxin Rev 7: 121–209.
- Bjarnason JB, Fox JW (1994): Hemorrhagic metalloproteinase from snake venoms. Pharmacol Ther 62: 325–372.
- Bjarnason JB, Tu AT (1978): Hemorrhagic toxins from Western diamondback rattlesnake (Crotalus atrox.) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin. Biochemistry 17: 3395–3404.
- Bode W, Huber R (2000): Structural basis of the endoproteinase-protein inhibitor interaction. Biochim Biophys Acta 1477: 241–252.
- Castro O, Gutierrez JM, Barrios M, Castro I, Romero M, Umana E (1999): Neutralization of the hemorrhagic effect induced by Bothrops asper. (Serpentes: Viperidae) venom with tropical plant extracts. Rev Biol Trop 47: 605–616.
- Chanhome L, Cox MJ, Wilde H, Jintakoon P, Chaiyabutr N, Sitprija V (1998): Venomous snakebite in Thailand I: Medically important snakes. Mili Med 163: 310–317.
- Dambisya YM, Lee TL, Gopalakrishnakone P (1994): Action of Calloselasma rhodostoma. (Malayan pit viper) venom on human blood coagulation and fibrinolysis using computerized thromboelastography (CTEG). Toxicon 32: 1619–1626.
- Devaraj T (1979): Bleeding manifestations in snakebite. Southeast Asian J Trop Med Public Health 10: 255–257.
- Gondachari TR, Nagarajan K, Pai BR (1956): Wedelolactone from Eclipta alba.. Tetrahedron Lett 15: 664–665.
- Gutierrez JM, Gene JA, Rojas G, Cerdas L (1985): Neutralization of proteolytic and hemorrhagic activities of Costa Rica snake venoms by a polyvalent antivenom. Toxicon 23: 887–893.
- Gutierrez JM, Avila C, Rojas E (1988): An alternative in vitro. method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 26: 411–413.
- Khin MM, Nyunt N, Khin MT (1978): The protective effect of Eclipta alba. on carbon tetrachloride-induced acute liver damage. Toxicol Appl Pharmacol 45: 723–728.
- Kini RM, Evans HJ (1992): Structural domains in venom proteins: Evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon 30: 265–293.
- Kondo H, Kondo S, Ikezawa H (1960): Studies on the quantitative method for determination of haemorrhagic activity of Habu snake venom. Jpn J Med Sci Biol 13: 43–51.
- Krishnaswamy NR, Seshadri TR, Sharma BR (1966): The structure of polythienyl from Eclipta alba.. Tetrahedron Lett 35: 4227–4230.
- Kunitz M (1947): Crystalline soybean trypsin inhibitor II: General properties. J Gen Physiol 30: 291–310.
- Laemmli UK (1970): Cleavage of structure protein during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
- Leal LKAM, Ferreira AAG, Bezerra GA, Matos FJA, Vina GSB (2000): Antinociceptive, anti-inflammatory and bronchodilator activities of Brazilian medicinal plants containing coumarin: A comparative study. J Ethnopharmacol 70: 151–159.
- Looareesuwan S, Viravan C, Warrell DA (1988): Factors contributing to fatal snake bite in the rural tropics: Analysis of 46 cases in Thailand. Trans R Soc Trop Med Hyg 82: 930–934.
- Markland FS (1998): Snake venoms and the hemostatic system. Toxicon 36: 1780–1800.
- Melo PA, Ownby CL (1999): Ability of wedelolactone, heparin, and para.-bromophenacyl bromide to antagonize the myotoxic effects of two crotaline venoms and their PLA2 myotoxins. Toxicon 37: 199–215.
- Melo PA, do Nascimento MC, Mors WB, Suarez-Kurtz G (1994): Inhibition of the myotoxic and haemorrhagic activities of crotalid venoms by Eclipta prostrata. (Asteraceae) extracts and constituents. Toxicon 32: 595–603.
- Melo PA, Almeida MAL, do Nascimento MC, Mors WB (1997): Ability of wedelolactone and its analogs to antagonize Bothrops venom activity. J Venom Anim Toxins 3: 184.
- Mors WB (1991): Plants active against snake bite. In: Wagner H, Farnsworth NR, eds., Economic and Medicinal Plant Research, Vol 5. London, Academic Press, pp. 353–373.
- Mors WB, do Nascimento MC, Parente JP, da Silva MH, Melo PA, Suarez-Kurtz G (1989): Neutralization of lethal and myotoxic activities of South American rattle snake venom by extracts and constituents of the plant Eclipta prostrata. (Asteraceae). Toxicon 27: 1003–1009.
- Ohsaka A (1979): Hemorrhagic, necrotizing and edema-forming effects of snake venoms. In: Lee CY, ed., Snake Venoms: Handbook of Experimental Pharmacology, Vol 5. Berlin, Springer-Verlag, pp. 480–546.
- Otero R, Nunez V, Jimenez SL, Fonnergra R, Osorio RG (2000): Snakebites and ethnobotany in the northwest region of Colombia Part II: Neutralization of lethal and enzymatic effects of Bothrops atrox. venom. J Ethnopharmacol 71: 505–511.
- Ouyang C, Hwang LJ, Huang TF (1983): α-Fibrinogenase from Agkistrodon rhodostoma. (Malayan pit viper) venom. Toxicon 21: 25–33.
- Pithayanukul P, Laovachirasuwan S, Bavovada R, Pakmanee N, Suttisri R (2004): Anti-venom potential of butanolic extract of Eclipta prostrata. against Malayan pit viper venom. J Ethnopharmacol 90: 347–352.
- Pithayanukul P, Ruenraroengsak P, Bavovada R, Pakmanee N, Suttisri R, Saen-oon S (2005): Inhibition of Naja kaouthia. venom activities by plant polyphenols. J Ethnopharmacol 97: 527–533.
- Ponnudurai G, Chung MCM, Tan NH (1993): Isolation and characterization of a hemorrhagin from the venom of Calloselasma rhodostoma. (Malayan pit viper). Toxicon 31: 997–1005.
- Reed L, Muench H (1938): A simple method of estimating 50 percent end point. Am J Hyg 27: 493–497.
- Reid HA, Chan KE (1968): The paradox in therapeutic defibrination. Lancet 9: 485–486.
- Tan NH, Kanthimathi MS, Tan CS (1986): Enzymatic activities of Calloselasma rhodostoma. (Malayan pit viper) venom. Toxicon 24: 626–630.
- Tan NH, Ponnudurai G, Chung MCM (1997): Proteolytic specificity of rhodostoxin, the major hemorrhagin of Calloselasma rhodostoma. (Malayan pit viper) venom. Toxicon 35: 979–984.
- Theakston RDG, Reid HA (1983): Development of simple standard assay procedures for the characterization of snake venom. Bull WHO 61: 949–956.
- Valente RH, Dragulev B, Perales J, Fox JW, Domont GB (2001): BJ45a, a snake venom metalloproteinase inhibitor. Eur J Biochem 268: 3042–3052.
- Viravan C, Looareesuwan S, Kosakarn W, Wuthiekanun V, McCarthy CJ, Stimson AF, Bunnag D, Harinasuta T, Warrell DA (1992): A national hospital-based survey of snakes responsible for bites in Thailand. Trans R Soc Trop Med Hyg 86: 100–106.
- Wagner H, Fessler B (1986): In vitro. 5-lipoxygenase hemmung durch Eclipta alba. extrakte und das coumestan derivat wedelolactone. Planta Med 52: 374–377.
- Wagner H, Geyer B, Kiso Y, Hikino H, Rao GS (1986): Coumestans as the main active principles of the liver drugs Eclipta alba. and Wedelia calendulacea.. Planta Med 52: 370–374.