Abstract
The current study was designed to investigate the effect of Citrus limon. (L.) Burm. (Rutaceae) fruits, commonly known as lemon, in experimental liver damage. The ethanol extract of Citrus limon. fruits was evaluated for its effects on experimental liver damage induced by carbon tetrachloride, and the ethyl acetate soluble fraction of the extract was evaluated on HepG2 cell line. The ethanol extract normalized the levels of aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), alkaline phosphatase (ALP), and total and direct bilirubin, which were altered due to carbon tetrachloride intoxication in rats. In the liver tissue, treatment significantly reduced the levels of malondialdehyde (MDA), hence the lipid peroxidation, and raised the levels of antioxidant enzymes superoxide dismutase (SOD) and catalase. It improved the reduced glutathione (GSH) levels in treated rats in comparison with CCl4-intoxicated rats. In the histopathologic studies, treated animals exhibited restoration of the liver architecture toward normal. Three doses of ethanol extract (i.e., 150, 300, and 500 mg/kg) were evaluated. The effect seen was dose dependent, and the effect of the highest dose was almost equal to the standard silymarin. In the investigation carried out on human liver–derived HepG2 cell line, significant reduction in cell viability was observed in cells exposed to CCl4. A dose-dependent increase in the cell viability was observed when CCl4-exposed HepG2 cells were treated with different concentrations of ethyl acetate soluble fraction of the ethanol extract. The highest percentage viability of HepG2 cells was observed at a concentration of 100 µg/mL. The extract merits further investigation to identify the active principles responsible for the hepatoprotective effect. The results from the current investigation also indicate good correlation between the in vivo. and in vitro. studies.
Introduction
Liver is the versatile organ of the body that regulates the internal chemical environment. Liver injuries induced by various hepatotoxins have been recognized as major toxicologic problems for years (Azer et al., Citation1997). In spite of tremendous strides in modern medicine, drugs are yet to be developed that protect the liver from damage induced by etiological agents, stimulate the functions of the liver, or help in regeneration of hepatic cells. Several potential hepatoprotective plants are used in traditional health care systems in India (Subramoniam & Pushpangadan, Citation1999). The National Innovation Foundation (NIF, Ahmedabad), established in 2000, documented about 30,000 herbal leads, from which some were selected for validation through scientific experiments. Citrus limon. fruit is one of those selected for the validation. Citrus limon. (L.) Burm.f. (Rutaceae), commonly known as lemon, is used in tribal medicine for the treatment of liver ailments and jaundice. Citrus limon. fruit is used to treat sluggish liver, rheumatism, fever and febrile diseases (Liebstein, Citation1927), diabetes (Gray & Flatt, Citation1997), as a refresher, a dietary supplement, antidysenteric, antispasmodic for gastralgia, and in colds and fever (De Feo et al., Citation1992). It is also used as an antiscorbutic, antispasmodic for colic, and an antiarthritic (Vazquez et al., Citation1997). The fruit juice is used to treat diabetes and high levels of cholesterol (De Feo et al., Citation1992). Citrus limon. contains a number of physiologically functional components such as citric acid, ascorbic acid, minerals, coumarins, limonoids, and flavonoids. Ascorbic acid (vitamin C) is an antioxidant reported to prevent reactive oxygen species (ROS)-mediated microsomal lipid peroxidation and protein degradation in both in vivo. and in vitro. studies. Lipid peroxidation and protein degradation are mediated by cytochrome P450 and are specifically prevented by ascorbic acid. Ascorbic acid prevents collagen oxidation and protects the mammalian tissues against oxidative damage both at the intracellular and extracellular levels (Chatterjee et al., Citation1995). The antioxidative flavonoids from lemon fruit have been studied and identified as eriocitrin and c-glucosyl flavones 6,8-di-c-β-glucosyl-diosmin (LE-B) and 6-c-β-glucosyl-diosmin (LE-C). Eriodictyol (aglycone of eriocitrin) has been shown to exhibit stronger activity than α -tocopherol in linoleic acid auto-oxidation and a rabbit erythrocyte membrane oxidation system (Miyake et al., Citation1997a). Eriocitrin and its metabolite were powerful antioxidants when tested on an in vitro. oxidation model for heart disease (Miyake et al., Citation1997b). Lemon flavonoid eriocitrin suppressed exercise-induced oxidative damage in rat liver (Minato et al., Citation2003). Eriocitrin in lemons suppressed oxidative stress when tested in streptozotocin (STZ)-induced diabetic rats (Miyake et al., Citation1998). Another bioflavonoid, hesperidin is reported to decrease the oxidative stress produced by carbon tetrachloride in rat livers and kidney (Tirkey et al., Citation2005). Naringin is a major flavonone glycoside of grapefruit and Citrus limon. and other Citrus. herbs. Naringin and naringenin are reported to inhibit nitrite-induced methemoglobin formation by scavenging superoxide, hydroxyl, and other free radicals (Kumar et al., Citation2003). Naringin suppressed lipopolysaccharide-induced tumor necrosis factor (TNF) release and liver injury in mice (Kawaguchi et al., Citation1999). Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. In this study, naringin was shown to have hepatoprotective action (Choe et al., Citation2001). In another study on cholesterol-fed rabbits, naringin exhibited antioxidant capacity based on increasing the gene expression of superoxide dismutase, catalase, and glutathione peroxidase (GSH-Px) and thereby increasing the hepatic superoxide dismutase and catalase activities, protecting the plasma vitamin E, and decreasing the hepatic mitochondrial H2O2 content (Jeon et al., Citation2001). Some coumarins (8-geranyloxypsolaren (LE-1), 5-geranyloxypsoralen (LE-2) and 5-geranyloxy-7-methoxycoumarin (LE-3)) are reported as unhibitors of in vitro. tumor promotion (chemopreventors) and superoxide and nitric oxide generation (Miyake et al., Citation1999). Studies cited above show that the constituents of plant and fruit are antioxidant, lipid peroxidation inhibitors, free-radical scavengers, chemopreventors, and hypocholesterolemic agents. Particularly, flavonoids are efficient antioxidants and lipid peroxidation inhibitors.
The peel and seed extracts of Citrus limon. are reported to have antioxidant activity using citronella oxidation system (Bocco et al., Citation1998). The seed extract of C. limon. is reported to have superoxide free radical scavenging activity in the superoxide produced by xanthine/xanthine oxidase (X/XOD) system (Orhan et al., Citation2003). The seed extract reduced the lipid peroxidation levels and increased the levels of superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), reduced glutathione (GSH), and glutathione-S.-transferase (GST) in the liver of male albino rats (Krishnamoorthy et al., Citation2003).
However, the biochemical basis and the mechanism of its effect on liver have not been scientifically studied. Hence, the current study was intended to investigate the in vitro. and in vivo. antihepatotoxic effects of the ethanol extract of the fruits of Citrus limon..
Materials and Methods
Materials
HepG2 cell line was obtained from the National Centre for Cell Sciences (NCCS; Pune, India). Minimum essential medium (MEM) was from Gibco BRL (California, USA) and fetal bovine serum (FBS) from HyClone (Utah, USA). Dimethyl sulfoxide (DMSO), trypsin, EDTA, antibiotic (1% penstrep) were obtained from Sigma Chemical Co. (St. Loius, MO, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from HiMedia (Mumbai, India). Diagnostic kits were purchased from Span Diagnostics Ltd. (Baroda, India). Carbon tetrachloride (extrapure) was obtained from Samir Tech-Chem Pvt. Ltd. (Baroda, India). Reduced glutathione and thiobarbituric acid was purchased from Kemphasol (Mumbai, India). Tris HCl was from Loba Chemie Pvt Ltd. (Mumbai, India). All other chemicals were obtained from SD Fine Chemicals (Mumbai, India).
Plant material
Fruits of Citrus limon. were collected in the winter season (January 2005) from Bhavnirger, Ahmedabad, India, and authenticated at the Department of Botany, Gujarat University, Ahmedabad, and a voucher specimen was placed in the Department of Pharmacognosy, L.M. College of Pharmacy, Ahmedabad (voucher no. 503).
Preparation of the extract
The fruits were dried under sun. The dried material was ground to coarse powder using a blender. The ethanol extract was prepared by simple maceration method. Dried powder (20 g) was extracted with ethanol (70%) (4 × 50 mL) for 24 h. The pooled extract was concentrated to dryness to get a semisolid mass (4.26%). The extract was stored in a glass container in a refrigerator until further use.
Fractionation of extract: For ethyl acetate soluble fraction (for investigation on HepG2 cell line), 10 g ethanol extract was dissolved in 20 mL water, extracted with 10 mL of ethyl acetate 3–4 times. All the ethyl acetate fractions were mixed in a porcelain dish and evaporated to dryness on a water bath.
Ethyl acetate soluble fraction (0.79%) was rich in flavonoids and phenolic compounds.
Preparation of drug
For in vivo. study, the ethanol extract of Citrus limon. suspended and solubilized in distilled water was freshly prepared before use, and this extract was given orally in different doses. Silymarin was suspended in sodium carboxy methyl cellulose (CMC) (0.3%) in distilled water. The doses are expressed as milligrams of dried extract per kilogram of rat.
For investigation on HepG2 cell line: Ethyl acetate soluble fraction of the ethanol extract of Citrus limon. was dissolved in MEM to obtain a stock solution of 1 mg/mL concentration and stored at −20°C prior to use. Further dilutions were made to obtain different concentrations ranging from 40 to 100 µg/mL with respective media and used for in vitro. investigations. A suspension of the standard powder silymarin was also prepared (100 µg/mL); silymarin powder was first dissolved in DMSO and then volume was made up to 10 mL with MEM, as it is not directly soluble in the media.
Hepatoprotective effect of the extract in carbon tetrachloride – induced liver damage
Healthy untreated Wistar rats of either sex weighing 200–250 g were used for the investigations. All the animals were maintained under standard husbandry conditions (22–28°C, 60–70% relative humidity, 12-h dark/light cycle) with food and water ad libitum.. The experimental procedures were approved by the institutional animal ethical committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (proposal no. 15/2005, LMCP, Ahmedabad).
The rats were divided into seven groups of six animals each. Group 1 served as normal control receiving only vehicle (distilled water, p.o.). Group 2 normal treated group received test drug extract 500 mg/kg, p.o., for 7 days.
Liver damage was induced by administration of CCl4 (1 mL/kg, i.p., as 50:50 solution in olive oil) every alternate day for 7 days, that is, on days 2, 4, 6 (3 applications) to animals of the remaining five groups.
Group 3 received only CCl4. Group 4 received CCl4 and standard reference silymarin 100 mg/kg, p.o., for 7 days. Group 5 received CCl4 and extract 150 mg/kg, p.o., for 7 days. Group 6 received CCl4 and extract 300 mg/kg, p.o., for 7 days. Group 7 received CCl4 and extract 500 mg/kg, p.o., for 7 days. Silymarin and test extract were administered daily for 7 days. After completion of the experimental period, the rats were fasted overnight and sacrificed by ether anesthesia. Blood and liver samples were collected for biochemical and histologic studies.
Assessment of liver function
Biochemical parameters aspartate aminotransferase (ASAT), alanine aminotransferase (ALAT), alkaline phosphatase (ALP), total bilirubin, direct bilirubin, total protein and albumin were assayed using diagnostic kits according to standard methods described elsewhere (Jendrassik & Grof, Citation1938; Lowry et al., Citation1951; Kind & King, Citation1954; Reitman & Frankel, Citation1957).
Antioxidant parameters or Anti–lipid peroxidation studies
Preparation of tissue homogenate
Liver tissue (1 g) was homogenized in 10 mL ice-cold Tris hydrochloride buffer (pH 7.2). The prepared homogenates were centrifuged and used for the determination of antioxidant parameters.
Post-mitochondrial supernatant preparation (PMS)
The homogenates were centrifuged at 800 × g. for 5 min at 4°C to separate the nuclear debris. The supernatant so obtained was centrifuged at 10,500 × g. for 20 min at 4°C to get the post-mitochondrial supernatant, which was used to assay catalase and superoxide dismutase (SOD) activity.
The malondialdehyde (MDA) level, a measure of lipid peroxidation, was assayed in the form of thiobarbituric acid reacting substances (TBARS) (Ohkawa et al., Citation1979). SOD level (Misra & Frodwich, Citation1972), catalase level (Abei, Citation1984), reduced glutathione (GSH) (Beutler et al., Citation1963), and the liver tissue protein (Lowry et al., Citation1951) were estimated.
Histopathologic studies
Paraffin sections (7 µm thick) of buffered formalin-fixed liver samples were stained with hematoxylin-eosin to study the liver histologic structure of the control and treated rats.
Hepatoprotective activity on HepG2 cell line
The screening of hepatoprotective activity was based on the protection of human liver–derived HepG2 cells against CCl4-induced damage (Ira et al., Citation1997; Vijayan et al., Citation2003) determined by estimating mitochondrial synthesis using tetrazolium assay (Ke et al., Citation1999; Bruggisser et al., Citation2002). HepG2 cells (from NCCS) were routinely grown and subcultured as monolayers in MEM supplemented with 10% fetal calf serum. The experiments in this investigation were conducted with cells that had been initially batch cultured for 10 days. At this stage, the cells were harvested and plated at approximately 2 × 105 cells/well in 96-well microtiter plates (Nunclon, Denmark) and placed in a humidified atmosphere of 5% CO2 at 37°C for 24 h. After 24 h period, when cells are in exponential growth phase, the cells were incubated for 1 h with 100 µL medium containing 1% CCl4 (toxicant), with or without various concentrations of the ethyl acetate soluble fraction of the ethanol extract (40 to 100 µg/mL) or the standard (100 µg/mL). Some cells are incubated with the fresh medium alone (as normal/control) (Vijayan et al., Citation2003). At the end of the period, the medium was removed and replaced with 100 µL fresh medium (washing step) so that there is no direct interaction between the test extract and MTT (Bruggisser et al., Citation2002). Then, cytotoxicity was assessed by estimating the viability of HepG2 cells by MTT reduction assay (Ke et al., Citation1999; Bruggisser et al., Citation2002). After the washing step, 10 µL MTT prepared in MEM without phenol red (MEM-PR) (5 mg/mL) was added in each well so that final concentration in each well is 0.5 mg/mL. The plates were gently shaken and incubated for 3 h at 37°C in a humidified 5% CO2 atmosphere. At the end of the period, 100 µL MTT solubilizing buffer (0.1 N HCL in propanol with 10% Triton-x 100) was added to each well and the plates were gently shaken to solubilize the blue-colored formazan. The plates were directly read on a microtiter plate reader SPECTRA MAX 190 (Molecular Devices, USA) at 540 nm.
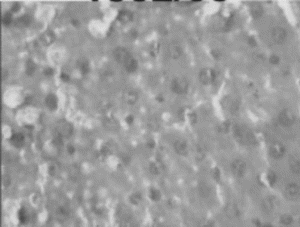
Figure 3 Ethanol extract (150 mg/kg) treated liver. Necrosis, Lymphocytic infiltration, occasional regenerating parenchymal cells.
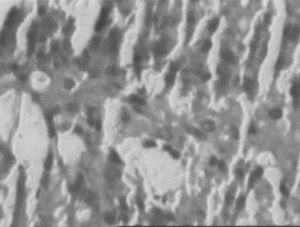
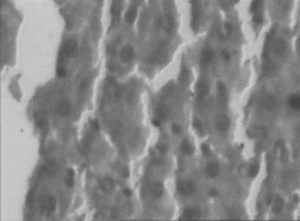
Figure 4 Ethanol extract (300 mg/kg) treated liver. Necrosis, inflammation and isolated liver cell regeneration.
Statistical analysis
Results are presented as mean ± SEM. Statistical difference between the means of the various groups was analyzed using one-way analysis of variance (ANOVA) followed by Tukey's multiple test. Data were considered statistically significant at p < 0.05. Statistical analysis was performed using Sigmastat statistical software.
Results
Hepatoprotective effects in carbon tetrachloride–induced liver damage
The effects of the ethanol extract of Citrus limon. fruits on carbon tetrachloride–intoxicated rats are recorded in Tables and . Intoxication of rats with CCl4 significantly altered the biochemical parameters when compared with normal control rats (p < 0.001), coupled with a marked hepatic oxidative stress. CCl4 challenge significantly (p < 0.001) decreased the levels of SOD and catalase in liver. Intracellular antioxidant GSH levels were also depleted. The levels of MDA, which is produced as a result of lipid peroxidation, were significantly (p < 0.001) increased.
Table 1.. Effect of treatment with the ethanolic extract of Citrus limon. fruits on biochemical parameters of CCl4 intoxicated rats
Table 2.. Effect of treatment with the ethanolic extract of Citrus limon. fruits on antioxidant parameters of CCl4 intoxicated rats
Treatment with the ethanol extractof Citrus limon. showed a significant decrease in ASAT, ALAT, ALP, total bilirubin, and direct bilirubin levels (p < 0.001) in serum when compared with CCl4-intoxicated rats. The effect is dose dependent; there is a good correlation between the dose of the extract and reduction in the increased levels of serum enzymes and total and direct bilirubin. The highest effect is seen with the dose of 500 mg/kg, and it was found almost equivalent to that of standard silymarin at 100 mg/kg. There was no significant difference seen in serum total protein and albumin levels between the normal group and CCl4-treated group and test drug and silymarin treated groups. Treatment also increased the levels of SOD and catalase in liver tissue significantly (p < 0.05). Improvement (p < 0.05) of hepatic GSH levels in treated rats in comparison with CCl4-intoxicated rats demonstrates the antioxidant effect of the extract. Treatment with the extract of Citrus limon. significantly (p < 0.05) reduced the levels of MDA indicating lipid peroxidation inhibiting effect of test drug. Standard silymarin at 100 mg/kg body weight also exhibited similar results. Improvement in the antioxidant parameters is also dose dependent.
The control treated animals showed similar biochemical profile as that of control (normal) animals. In liver tissue, there was increase in the levels of SOD, catalase, and GSH and slight decrease in MDA levels compared with normal animals, however not statistically significant; these results indicate that the extract in normal animals increased antioxidant enzymes and GSH as well as reduced lipid peroxidation.
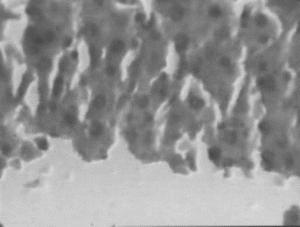
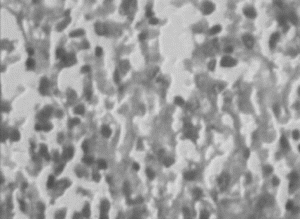
In the histopathologic studies, normal animals showed central vein and normal liver parenchymal cells. The CCl4-intoxicated animals showed extensive necrosis, inflammation, and infiltration by lymphocytes. In the treated groups at the lowest dose (150 mg/kg), necrosis, sparse lymphocytic infiltration, and occasional regenerating parenchymal cells were seen. With 300 mg/kg dose, there was isolated liver cell regeneration around the necrotic focus with reduced necrosis and inflammation. While with the highest dose (500 mg/kg), areas of regeneration were observed, there was a greater amount of regeneration with mild inflammation and some lymphocyte infiltration in the necrotic area. Standard silymarin-treated animals showed greater number of regenerating liver cells around the necrotic area. Thus in the histopathologic studies also the effect is dose dependent, and the effect of the highest dose is very significant and similar to that of the standard silymarin.
Hepatoprotective effects in the HepG2 cell line
The CCl4-exposed HepG2 cells showed a percentage viability of 8%. These exposed cells, when treated with different concentrations of the ethyl acetate soluble fraction of the ethanol extract of Citrus limon., showed a dose-dependent increase in percentage viability, and the results were highly significant (p < 0.001) when compared with CCl4-exposed cells. The percentage viability ranged between 28% and 83% at 40 to 100 µg/mL concentration of the ethyl acetate soluble fraction of the ethanol extract of Citrus limon. (). The increase in percentage viability of the HepG2 cells treated with ethyl acetate soluble fraction at 100 µg/mL was more potent than that produced by the standard silymarin at 100 µg/mL.
Table 3. Effect of the ethyl acetate soluble fraction of ethanolic extract of C. limon. fruits on CCl4 intoxicated HepG2 cells
Discussion
The current study reveals the hepatoprotective effect of Citrus limon. against carbon tetrachloride–induced toxicity in animal model and HepG2 cells in culture.
Carbon tetrachloride has been found to induce extensive liver damage within 24 h after intraperitoneal administration. As a result of this, accumulation of fat in the liver and necrosis in the centrilobular region of the liver occurs. CCl4 is metabolized in the liver by cytochrome P450 to trichloromethyl radical (), which reacts with GSH to form a GSH-containing radical and causes various pathologic and toxicologic manifestations. The trichloromethyl radical (
) rapidly reacts with molecular oxygen yielding the trichloromethylperoxyl radical (
), a highly reactive species. These free radicals initiate the damaging process through covalent binding to cell macromolecules (
), enhancement of membrane lipid peroxidation (
), and, in the second step, derangement of intracellular free calcium homeostasis. Early events associated with the cytotoxicity of CCl4 after activation includes lipid peroxidation and covalent binding to proteins and lipids followed immediately by diminished protein and lipid turnover and alterations in calcium homeostasis. At the molecular level, CCl4 activates tumor necrosis factor (TNF)-α, nitric oxide (NO), and transforming growth factors (TGF)-α and -β in the cell, processes that appear to direct the cell primarily toward (self-) destruction or fibrosis (Weber et al., Citation2003).
Treatment with the ethanol extract of Citrus limon. exhibited significant restoration of the altered biochemical parameters toward normal in CCl4-intoxicated rats. In the histopathologic studies, treated animals exhibited restoration of the liver architecture toward normal. The effect of ethanol extract at 500 mg/kg was comparable with standard silymarin at 100 mg/kg dose. The effect was dose dependent with highest protection at 500 mg/kg dose.
CCl4 intoxication significantly increased the lipid peroxidation levels in the liver. Treatment with the ethanol extract of Citrus limon. significantly reduced the lipid peroxidation levels compared with CCl4-intoxicated animals. The treatment increased the levels of antioxidant enzymes SOD and catalase and that of the cellular antioxidant GSH. These results indicate a potent antioxidant and lipid peroxidation inhibiting activity of the extract. Our results are in line with the reported antioxidant and lipid peroxidation inhibiting activity of the peel and seed extracts of the fruit (Bocco et al., Citation1998; Orhan et al., Citation2003; Krishnamoorthy et al., Citation2003).
The investigation carried out in human liver–derived HepG2 cells against CCl4-induced damage also confirmed the hepatoprotective nature of the extract.
The results from the current study indicate a good correlation between in vivo. and in vitro. studies. They indicate that Citrus limon. ethanol extract is effective against hepatic injury induced by carbon tetrachloride.
Our findings support the reported therapeutic use of this fruit in tribal medicine for liver ailments and jaundice.
Various flavonoids are reported to be present in the Citrus limon. fruit, and they might be playing a contributory role toward the hepatoprotective action and antioxidant potential. Another important constituent is vitamin C. Vitamin C together with flavonoids enhances the effectiveness of flavonoids, and flavonoids prevent oxidation of vitamin C and enhance its absorption and effectiveness. This might be important in the effectiveness of the extract.
The probable mechanism of action of the observed effects appears to be activity as an antioxidant, free-radical scavenger, inhibition of lipid peroxidation, inhibition of superoxide and nitric oxide generation, superoxide scavenger, and increasing the level of antioxidant enzymes and intracellular antioxidant GSH. Other mechanism could be inhibition of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and inhibition of TNF-α release by naringin and naringenin (Kawaguchi et al., Citation1999; Choe et al., Citation2001)
Further work on other models with other hepatotoxicants, is suggested. Further work is also suggested for finding specific constituent(s) responsible for hepatoprotective activity and also to discover the exact mechanism of action.
Acknowledgments
We thank Dr. B.B. Lohray, Dr. Sanjeevkumar, and Dr. Ashish Goel, Zydus Research Centre, Ahmedabad, for providing HepG2 cell line and facilities for work.
References
- Aebi H (1984): Catalase in vitro.. Methods Enzymol 105: 121–126.
- Azer SA, McCaughan GM, Stacey NH (1997): Liver and Environmental Xenobiotic. New Delhi, Narosa Publishing House, pp. 178–180.
- Beutler E, Duron O, Kelly D (1963): Improved method of determination of blood glutathione. J Lab Clin Invest 61: 882–888.
- Bocco A, Cuvelier ME, Richard H, Berset C (1998): Antioxidant activity and phenolic composition of Citrus. peel and seed extracts. J Agric Food Chem 46: 2123–2129.
- Bruggisser R, Daeniken KV, Jundt G, Schaffner W, Reinert HT (2002): Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med 68: 445–448.
- Chatterjee IB, Mukhopadhyay CK, Ghosh MK (1995): Vitamin C: A potential savior against free radical-induced oxidative damage. Curr Sci 69: 747–751.
- Choe SC, Kim HS, Jeong TS, Bok SH, Park YB (2001): Naringin has an antiatherogenic effect with the inhibition of intercellular adhesion molecule-1 in hypercholesterolemic rabbits. J Cardiovasc Pharmacol 38: 947–955.
- De Feo V, Aquino R, Menghini A, Ramundo E, Senatoare F (1992): Traditional phytotherapy in the Peninsula sorrentina, Campania, southern Italy. J Ethnopharmacol 36: 113–125.
- Gray AM, Flatt PR (1997): Nature's own pharmacy: The diabetes perspective. Proc Nutr Soc 56: 507–517.
- Ira TM, Hughes RD, McFarlane IG (1997): Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Ethnopharmacol 49: 1132–1135.
- Jendrassik L, Grof P (1938): The method of determination of bilirubin with photoelectric colorimeter. Biochem J 297: 81–89.
- Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, Rhee SJ, Choi MS (2001): Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci 69: 2855–2866.
- Kawaguchi K, Kikuchi S, Hasegawa H, Maruyama H, Morita H, Kumazawa Y (1999): Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur J Pharmacol 368: 245–250.
- Ke H, Hisayoshi K, Aijiun D, Yongkui J, Shigeo I, Xinsheng Y (1999): Antineoplastic agents III: Steroidal glycoside from Solanum nigrum.. Planta Med 65: 35–38.
- Kind PR, King EJ (1954): Estimation of plasma phosphate by determination of hydrolysed phenol with aminopyrines. J Clin Pathol 7: 322–330.
- Krishnamoorthy P, Vainathan S, Ravikumar S, Parakathullah KM (2003): Effect of lemon seed Citrus limon. extract on antioxidant enzymes in liver of male albino rats. Proceedings of First National Interactive Meet on Medicinal and Aromatic Plants 1: 299–301.
- Kumar MS, Unnikrishnan MK, Patra S, Murthy K, Srinivasan KK (2003): Naringin and Naringenin inhibit nitrite-induced methemoglobin formation. Pharmazie 58: 564–566.
- Liebstein AM (1927): Therapeutic effects of various food articles. Am Med 33: 33–38.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Minato K, Miyake Y, Fukumoto S, Yamamoto K, Kato Y, Shimomura Y, Osawa T (2003): Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci 72: 1609–1616.
- Misra H, Frodwich I (1972): The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. Biol Chem 247: 3170–3175.
- Miyake Y, Yamamoto K, Osawa T (1997a): Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon. Burm. F.) and its antioxidative activity. Food Sci Technology Int 3: 84–89.
- Miyake Y, Yamamoto K, Morimitsu Y, Osawa T (1997b): Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J Agric Food Chem 45: 4619–4623.
- Miyake Y, Yamamoto K, Tsujihara N, Osawa T (1998): Protective effects of lemon flavonoids on oxidative stress in diabetic rats. Lipids 33: 689–695.
- Miyake Y, Murakami A, Sugiyama Y, Isobe M, Koshimizu K, Ohigashi H (1999): Identification of coumarins from lemon fruit (Citrus limon.) as inhibitors of in vitro. tumor promotion and superoxide and nitric oxide generation. J Agric Food Chem 47: 3151–3157.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Orhan I, Aydin A, Colkesen A, Sener B, Isimer AI (2003): Free radical scavenging activities of some edible fruit seeds. Pharm Biol 41: 163–165.
- Reitman S, Frankel S (1957): A colorimetric method for the determination of serum oxaloacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28: 56–63.
- Subramoniam A, Pushpangadan P (1999): Development of phytomedicine for liver diseases. Indian J Pharmcol 31: 166–175.
- Tirkey N, Pilkhwal P, Kuhad A, Chopra K (2005): Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol 52: 1471–2210.
- Vijayan P, Prashant HC, Vijayraj P, Dhanaraj SA, Badami S, Suresh B (2003): Hepatoprotective effect of the total alkaloid fraction of Solanum pseudocapsicum. leaves. Pharm Biol 41: 443–448.
- Vazquez FM, Sauarez MA, Perez A (1997): Medicinal plants used in the Barros area, Badajoz Province (Spain). J Ethnopharmacol 44: 81–85.
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes (2003): Carbon tetrachloride as a toxicological model. Crit Rev Toxicol 33: 105–136.