Abstract
Six methanol extracts from different parts of plants used in northeast Mexico as general health supplements were examined for their potential as antioxidants. The plants evaluated were: Turnera diffusa. Wild. (Turneraceae), Cucurbita. foetidissima Kunth (Cucurbitaceae), Flourensia cernua. D.C. (Asteraceae), Selaginella pilifera. A. Braun (Selaginellaceae), Juglans mollis. Engelm. (Juglandaceae) and Centaurea americana. Nutt. (Asteraceae alt. Compositae). Antioxidant properties of these extracts were evaluated by means of different assays, including the l,l-diphenyl-2-picrylhydrazyl radical test by TLC and spectrophotometry, inhibition of xanthine oxidase (XO) activity, and total phenolics content. Five plants showed high scavenging potential; their total phenolics content was also high. The extracts from four plants inhibited the activity of XO. Two of the most promising plants, T. diffusa. and J. mollis., did not show cytotoxicity. Considering that antioxidants prevent lipid peroxidation in foods and help in the treatment and prevention of degenerative illness, these two species are good candidates to be considered and further evaluated as natural additives in foods to provide protection against oxidative degradation.
Introduction
Antioxidants, as scavengers of free radicals, have been widely used as food additives to prevent lipid peroxidation. Moreover, they are known to reduce the risks of certain types of cancer and many chronic degenerative diseases, such as coronary heart diseases, cardiovascular diseases, and aging. Epidemiologic studies have indicated that the dietary intake of antioxidant substances is inversely associated with mortality from these degenerative processes (Venereo, Citation2002; Velásquez et al., Citation2004; Zollo et al., Citation2004). Synthetic antioxidants have been widely used in the food industry, but some have been restricted because of side effects, such as liver damage and carcino-genicity. Therefore, many vegetables have become attractive to scientists as natural sources of antioxidants that could be safer than synthetic sources. Evaluation of antioxidant and antiradical activities of plant products cannot be carried out accurately by any single universal method. There are numerous methods for evaluating antioxidant activity (Imeh & Khokhar, Citation2002; Kaur & Kapoor, Citation2002; Hu et al., Citation2003; Picerno et al., Citation2003; Shon et al., Citation2003; Mikhaeil et al., Citation2004). Among these, the measurement of radical scavenging activity using stable free radicals, such as l,l-diphenyl-2-picrylhydrazyl (DPPH), has been widely used (Shon et al., Citation2003); moreover, the quantification of total phenolics generally agrees with the results obtained from DPPH scavenging assays (Imeh & Khokhar, Citation2002; Kaur & Kapoor, Citation2002; Picerno et al., Citation2003). The inhibition of xanthine oxidase (XO), a free radical-producing enzyme, has also been used to predict antioxidant activity (Russo et al., Citation2002). The plant kingdom represents great potential for new molecules that await discovery, and more than 90% of plant species have not been exhaustively investigated (Ortholand & Ganesan, Citation2004). Mexico has around 50,000 species of plants. Specifically, northeastern Mexico has a semiarid climate and displays a great variety of wild plants that can grow under extreme climatic conditions (Adame & Adame, Citation2000). Hence, in this article, we have examined the antioxidant activities of the extracts obtained from six plants growing in our region that have been traditionally used as general health supplements. The plants considered are the following: (1) Turnera diffusa. Wild. (Turneraceae), reported as being an aphrodisiac since Mayan times (Martinez, Citation1989; Arletii et al., 1999), as well as being an expectorant, tonic, and diuretic (Godoi et al., Citation2004). Several essential oils have been isolated from this plant (Alcaraz et al., Citation2004), as well as flavonoids and arbutin (Piacente et al., Citation2002). (2) Flourensia cernua. D.C. (Asteraceae), which has been used as an anti-inflammatory (Martinez, Citation1989; Argueta et al., Citation1994; Arletii et al., 1999; Godoi et al., Citation2004) and to prevent indigestion (Tellez et al., Citation2001). Several compounds, such as dehy-drofluorensic acid, fluorensadiol, methyl orsellinate, ermanin, tetracosan-4-olide, pentacosan-4-olide, hexacosan-4-olide, heptacosan-4-olide, octacosan-4-olide, nona-cosan-4-olide, and triacontan-4-olide, have also been isolated (Mata et al., Citation2003). (3) Centaurea americana. Nutt. (Asteraceae alt. Compositae), which has been used by traditional healers to protect the liver. (4) Cucurbita foetidissima. Kunth (Cucurbitaceae), the fruits of which alleviate pain in rheumatic areas (Curtin, Citation1947). (5) Selaginella pilifera. A. Braun (Selaginellaceae), which is used for gastrointestinal diseases and colic (Bye, Citation1985). (6) Juglans mollis. Englem. (Juglandaceae), the leaves of which are used for rheumatism, cicatrization, and leucorrhea (Casas et al., Citation1994).
Materials and Methods
Reagents
DPPH, Folin-Ciocalteu reagent, quercetin, pirocatechol, xanthine, XO, Roswell Park Memorial Institute (RPMI) 1640 medium, and Trypan blue were purchased from Sigma Chemicals Co. (St Louis, MO, USA). All other reagents were of analytical grade. Chromatographic plates were silica gel 60 F240 (Merck, Darmstadt, Germany).
Plant material
All plants were collected from the field. Collections were carried out in October 2000, July 2002, and in the spring and summer of 2003 and 2004 from different regions in the states of Nuevo Leon and Coahuila (). Different parts of the plants were collected and treated independently. Voucher specimens for each plant were no deposited at the herbarium of the Facultad de Ciencias Biologicas, Universidad Autónoma de Nuevo León (see ).
Table 1 Plant species from northeast Mexico screened for antioxidant activity.
Preparation of extracts
Air-dried samples (l0 g) were ground into a fine powder in a mill and extracted with 50 mL methanol three times. The solvent was removed using a rotary evaporator at 37°C under reduced pressure to obtain a dry extract. The extracts were stored at 4°C until used.
DPPH radical scavenging activity
DPPH radical scavenging activity was assessed in two ways (Shon et al., Citation2003). Ten microliters of each extract (1 mg/mL in ethanol) was applied to a chromatographic plate. The chromatograph was developed using ethyl acetate:acetic acid:formic acid:water (100:11:11:27) as an eluent. The plate was developed by means of a DPPH solution (2 mg/mL in ethanol); 30 min later, the color of the spots was determined visually. The quantitative evaluation of DPPH scavenging activity was carried out spectrophotometrically in a Beckman DU 7500 (Beckman Coulter, Inc., Fullerton, CA, USA) spectro-photometer as follows: 500 μL of each extract (1 mg/mL in ethanol) was added to a 500 μL solution of DPPH (0.2mg/mL in ethanol), shaken, and allowed to stand at room temperature in darkness for 20 min. The absorbance was then measured at 517 nm. For both assays, quercetin was used as a positive control. The capacity to scavenge the DPPH radical was calculated as follows:
where A. is the absorbance of the negative control (DPPH plus ethanol) and B. is the absorbance of the sample (DPPH, ethanol plus sample).
Determination of total phenolics content
The total amount of soluble phenolics in each extract was determined according to a previously described spectrophotometric assay (Kaur & Kapoor, Citation2002). Briefly, 100 μL of each extract (1 mg/mL in ethanol) was added to 3 mL distilled water and 0.5 mL Folin-Ciocalteu reagent. The mixture was shaken and added to 2 mL of a sodium bicarbonate solution (20% p/v). After shaking, the mixture was allowed to stand at room temperature for 60 min. The absorbance was then measured at 650 min. Pirocatechol was used as a positive control. The total phenolics content was expressed as grams of pirocatechol per 100 g sample according to the following equation:
where A. is the absorbance obtained from pirocatechol and B. is the absorbance obtained from the sample.
Determination of XO activity inhibition
This assay was undertaken by following the formation of uric acid at 292 nm, as previously described by Russo et al. (Citation2002). Briefly, different concentrations of the extracts in ethanol (10–150 μg/mL) were mixed with 24 mU of a XO solution (25 mM) in phosphate buffer (50 mM, pH 7.8). Results are expressed as percent of enzymatic inhibition. Quercetin, in a range between 10 and 150 μg/mL, was used as a positive control.
Cytotoxicity
The selected extracts (5, 50, 250, and 2500 μg/mL) in DMSO were added to primary cultures of mononuclear cells (200,000 cells/well) obtained from spleens of Balb/c mice in 200 μL of RPMI 1640 medium. A growing control and a growing control with DMSO were used. After 72 h incubation, the cells were dyed with Trypan blue and their viability observed under a microscope (Mosmann, Citation1983).
Statistical analysis
All experiments were carried out in triplicate, and results were expressed as means and 2 standard deviations. The results were examined with a Student's t.-test (p ≤ 0.025) to determine their reproducibility.
Results and Discussion
Scavenging of DPPH radicals
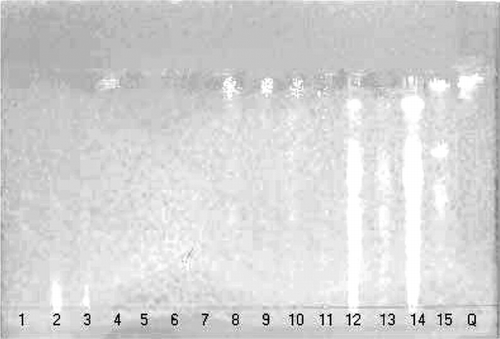
The DPPH radical scavenging assay is commonly employed to evaluate the ability of antioxidants to scavenge free radicals. In the current article, this test was made qualitatively by means of TLC; the plates were developed with the DPPH reagent. Active fractions, as well as the positive control, could be observed as clear spots that contrasted with the purple background of the plate (). The greatest activity was found in the extracts obtained from the leaves, bark, and fruits of J. mollis. and the flowers of C. americana.. For these extracts, several spots were observed with intensities similar to that displayed by the control (quercetin). The extracts obtained from the stems and roots of T. diffusa., the stems, roots, and flowers of F. cernua., and the leaves of S. pilifera. displayed only one active spot with an intensity lower than that observed for the control. The extracts obtained from leaves of T. diffusa., the leaves, roots, and stems of C. foetidissima., and the leaves of F. cernua. did not show any active spot. Furthermore, the results obtained from the spectrophotometric assay confirmed the observations made by means of TLC (). Of the 15 extracts assayed, 10 (almost 70%) displayed activity equal to or higher than quercetin; all the extracts obtained from J.. mollis., as well as the extracts obtained from the stems and roots of T. diffusa. and F. cernua., showed values higher than that obtained for quercetin, whereas the extracts obtained from the leaves of T. diffusa. and the flowers of C. americana. showed results similar to the control. This finding shows that both the TLC and spectrophotometric methods are comparable and reliable. Although TLC is a qualitative test, it has the advantage of being rapid and simple as it permits the determination of a great number of samples at the same time. Moreover, it is adequate to follow a biologically guided fractionation of the extracts.
Table 2 Antioxidant activity of 15 plant extracts.
Total phenolics content
The total phenolics content, as estimated by the Folin-Ciocalteu assay, ranged from 0.8 to 23 g of pirocatechol/ 100 g extract (). A good agreement could be found between these results and those obtained by the DPPH scavenging method. The greatest phenolics content was obtained with the extracts of J. mollis., as well as with the stems and leaves of T. diffusa. and the stems and roots of F. cernua.. These findings suggest that phenolics could be responsible for the antioxidant activity displayed by these extracts. Furthermore, it is interesting to note that the total phenolics content in these extracts was high compared with the values generally reported by other authors for different plant materials (Wang et al., Citation1997; Mosca et al., Citation2000; Imeh & Khokhar, Citation2002; Kaur & Kapoor, Citation2002; Georgè et al., Citation2005).
XO activity inhibition
Thirteen of the 15 extracts under evaluation (86%) produced inhibition of the enzyme, XO, in concentrations ranging from 10 to 100 μg/mL (). The most active extracts at 10 μg/μL were those obtained from the roots of T. diffusa., the bark of J. mollis., and the leaves of C. foetidissima.. Extracts that did not show any activity at this concentration were tested at greater concentrations. Quercetin was assayed at all concentrations; however, only the concentration that displayed 100% inhibition is reported. F. cernua. extracts did not show any inhibition, even at the greatest concentration tested. It is of interest that C. foetidissima. extracts displayed strong enzymatic inhibition; however, the total phenolics content, as well as DPPH activity, was very poor. These findings suggest that the antioxidant compounds present in this plant could act through a single electron transfer reaction rather than a hydrogen transfer reaction, which is the mechanism that evaluates the DPPH test (Huang et al., Citation2005; Prior et al., Citation2005).
Cytotoxicity
Based on the results obtained, four extracts were considered as good candidates for use as food additives and in a follow-up bioassay-guided fractionation: the 260 extracts obtained from the leaves and stems of T. diffusa. and those from the bark and leaves of J. mollis.. These extracts did not show toxicity on a lymphocyte cell line at a concentration of 2.5 mg/mL.
In conclusion, in the current study, 15 extracts were evaluated for their antioxidant activity, most of which showed activity in at least one of the tests used. Moreover, the extracts obtained from J. mollis. and T. diffusa. showed activity comparable with or greater than that of the control used. According to our results, the compounds responsible for the antioxidant activity in these two plants could be phenolics. It should also be noted that these extracts did not show cytotoxicity. Therefore, they could be considered as additives to foods with the purpose of preventing oxidation and/or to provide benefits to general health. In fact, T. diffusa. is used as a liquor in Mexico and is also found among the ingredients of a sauce used for seafood. A bioassay-guided fractionation of these two plants is now in progress to isolate and characterize the compounds responsible for their activity.
Acknowledgments
The authors wish to gratefully acknowledge PROMEP, Mexico (103.5/04/2590) and CONACYT-Mexico (grant SALUD-2002-CO1-7274) for financial support. The authors are grateful to the biologist Humberto Sánchez Vega for help in the collection and identification of plants and to Marco Antonio Guzmán Lucio and Maria del Consuelo González de la Rosa for final taxonomic identification of all the species reported here. Ivonne Carrera is acknowledged for her technical assistance in the extraction procedure.
References
- Adame J, Adame H. Plantas Curativas del Noreste Mexicano. 2000; 11–15, México, D. F., Ed. Castillo
- Alcaraz L, Delgado J, Real S. Analysis of essential oils from wild and micropropagated plants of damiana (Turnera diffusa). Fitoterapia 2004; 75: 696–701
- Argueta V A, Cano A LM, Rodarte M E. Atlas de las Plantas Medicinales de la Medicina Traditional Mexicana, Vol 1. México, D. F. Institute Nacional Indigenista. 1994; 51
- Arletti R, Benelli A, Cavazzuti E, Scarpetta G, Bertolini A. Stimulating property of Turnera diffusa. and Pfaffia paniculata. extracts on the sexual behavior of male rats. Physicopharmacology 1999; 1: 15–19
- Bye R. Medicinal plants of the Tarahumara Indians of Chihuahua, Mexico. Two Mummies from Chihuahua: A Multidisciplinary Study, R A Tyson, D V Elerick, 1985; 77–104, San Diego, San Diego Museum Papers, No. 19
- Casas A, Viveros J L, Caballero J. Etnobotánica Mixteca. Sociedad, Cultura y Recursos Naturales en la Montaña de Guerrero. 1994; 366, Mexico, D.F., INI-CONACULTA
- Curtin L SM. Healing Herbs of the Upper Rio Grande. Laboratory of Anthropology, Santa Fe, NM 1947; 31–33
- Georgé S, Brat P, Alter P, Amiot M J. Rapid determination of polyphenols and vitamin C in plant-derived products. J Agric Food Chem 2005; 53: 1370–1373
- Godoi A F, Vilegas W, Godoi R H, Van Vaeck L, Van Grieten R. Application of low pressure gas chromatography-ion trap mass spectrometry to the analysis of the essential oil of Turnera diffusa. (Ward) Urb. J Chromatogr A 2004; 1027: 127–130
- Hu Y, Xu J, Hu Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis. Millar) extracts. J Agric Food Chem 2003; 51: 7788–7791
- Huang D, Ou B, Prior R L. The chemistry behind antioxidant capacity assays. J Agric Food Chem 2005; 53: 1841–1856
- Imeh U, Khokhar S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J Agric Food Chem 2002; 50: 6301–6306
- Kaur C, Kapoor H C. Antioxidant activity and total phenolic content of some Asian vegetables. Int J Food SciTech 2002; 37: 153–161
- Martinez M. Las Plantas Medicinales de Mexico. D. F., Ediciones Betas, México 1989; 119, 186
- Mata R, Byeb R, Linares E, Macias M, River I, Perez O, Timmermann B N. Phytotoxic compounds from Flourensia cernua. Phytochemistry 2003; 64: 285–291
- Mikhaeil B R, Badria F A, Maatooq G T, Amer M M. Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch 2004; 59: 468–476
- Mosca L, Marco C, Visioli F, Cannella C. Enzymatic assay for the determination of olive oil polyphenol content: Assay conditions and validation of the method. J Agric Food Chem 2000; 48: 297–301
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J Immunol Methods 1983; 65: 55–63
- Ortholand J Y, Ganesan A. Natural products and combinatorial chemistry: Back to the future. Curr Opin Chem BiolS 2004; 271–280
- Piacente S, Camargo E ES, Zampelli A, Gracioso J S, Souza A R, Pizza C, Vilegas W. Flavonoids and arbutin from Turnera diffusa. Z Naturforsch 2002; 57c: 983–985
- Picerno P, Mencherini T, Lauro M R, Barbate R, Aquino R. Phenolic constituents and antioxidant properties of Xanthosoma violaceum. leaves. J Agric Food Chem 2003; 51: 6423–6428
- Prior R L, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005; 53: 4290–4302
- Russo A, Longo R, Vanella A. Antioxidant activity of propolis: Role of caffeic acid phenethyl ester and galangin. Fitoterapia 2002; 73: S21–S29
- Shon D H, Kim Y C, Oh S H, Park E J, Lee B H. Hepa-toprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine 2003; 10: 165–169
- Tellez M, Estell R, Fredrickson E, Powell J, Wedge D, Schrader K, Kobaisy M. Extracts of Flourensia cernua. (L.): Volatile constituents and antifungal, anti-algal, and antitermite bioactivities. J Chem Ecol 2001; 27: 2263–2273
- Velásquez M, Gómez B, Contreras R. El envejecimiento y los radicales libres. Ciencias 2004; 75: 36–43
- Venereo J R. Daño oxidativo, radicales libres y antioxidantes. Rev Cubana Med Milit 2002; 31(2)126–133
- Wang C, Lee W, Peng C. Contents of phenolics and alkaloids in Areca catechu. Linn, during maturation. J Agric Food Chem 1997; 45: 1185–1188
- Zollo A, Furno M, Grandola G, Totáro M, Aloj E. Factores ambientales generadores de radicales libres y factores clinico-sanitarios y ocupacionales de riesgo de irradiaciones: preventión y protectión. Hig Sanid Ambient 2004; 4: 65–71