Abstract
Thalia geniculata. L. (Marantaceae) is an African medicinal plant traditionally used in Benin to treat malaria and other parasitic diseases. There is little ethnobotanical and almost no chemical information available for this species. The phytochemical analysis of the aerial parts of the plant led to the isolation of five compounds, identified as sitoindoside I [β-sitosterol-(6-O.-hexadeca-noyl)-3-O.-β-d-glucoside] (1), daucosterol β-sitosterol-3-0-β-d-glucoside (2), stigmasterol (3), β-sitosterol (4), and geranylfarnesol (5). The structural elucidation was achieved using spectrometric methods and by comparison with the literature. Biological activity was evaluated against Plasmodium falciparum, Trypanosoma rhodesiense, Trypanosoma cruzi.,and Leishmania donovani.. Geranylfarnesol (5) showed significant antiprotozoal activity against P. falciparum. and L. donovani., with IC50 values of 12.7 and 13.2 μM, respectively.
Introduction
Protozoal diseases, such as malaria, trypanosomiasis, and leishmaniasis, are a major threat for public health. Every year, malaria causes 300 to 500 million clinical cases and more than 1 million deaths, and there is increasing prevalence of malaria exhibiting resistance of Plasmodium falciparum. to inexpensive, cheap standard treatments (Wellems & Plowe, Citation2001). Leishmaniasis and trypanosomiasis are major causes of mortality and cause much economic hardship, particularly in the developing world. Unfortunately, there are few drugs available to treat these two diseases, and most of these drugs suffer from poor clinical efficacy and unwanted effects. There is an urgent need to discover new therapeutic agents for these parasitic diseases, and traditional medicine knowledge can be useful to open new ways in the field of antiprotozoal therapy. In a previous work, we reported in vitro. anti-plasmodial activity of Thalia geniculata. L. (Marantaceae), a traditional medicinal shrub of Benin used against malaria in folk medicine, toward both chloroquine-sensitive and chloroquine-resistant strains of P. falciparum. (Adjanohoun et al., Citation1989; Weniger et al., Citation2004). In this study, we report the isolation and identification of five compounds from T. geniculata. aerial parts and their evaluation for anti-trypanosomal (Trypanosoma brucei rhodesiense. STIB 900 strain; T. cruzi. Tulahuen C4 strain), leishmanicidal (Leishmania donovani. MHOM-ET-67/L82 strain), and antiplasmodial (P. falciparum. Kl multidrug-resistant strain) activities.
Materials and Methods
Plant material
The collection of aerial parts of T. geniculata. was carried out during January 2005 in the area of Godomey, Department of the Atlantic (southern Benin). Botanical determination was performed by taxonomists from the Herbier National of Abomey-Calavi University of Cotonou in Benin, and voucher specimens were deposited at the same herbarium. The aerial parts were air-dried, powdered (0.2 mm sieve), and subjected to extraction with methanol.
Extraction and isolation
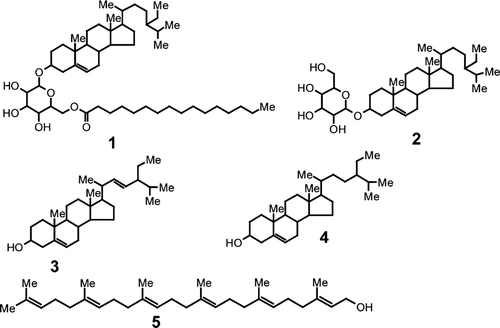
Dry powdered aerial parts of T. geniculata. (160 g) were extracted with methanol by maceration at room temperature during 72 h. The dried methanol extract (29.27 g) was partitioned between water and methylene chloride. The methylene chloride fraction was concentrated to dryness and chromatographed by gradient elution on an open column (silica gel Si 60, 230–400 mesh) using methylene chloride and methanol. Four fractions (A, 80 mg; B, 110 mg; C, 170 mg; and D, 60 mg) were obtained and purified using recrystallization or low-pressure chromatography (Horizon Pump, Biotage Inc., Uppsala, Sweden; 12 × 75 mm Flash 12 silica gel 40–63 (am cartridges). Compound 5 (26 mg) () was obtained from fraction A using low-pressure chromatography with a binary mixture of solvent made up with cyclohexane and methylene chloride in the proportions 30:70. Fraction B, soluble in methylene chloride, was purified by recrystallization with methanol. The white precipitate obtained was purified by successive washing with methanol to obtain 26 mg of a mixture of 3 and 4 in the proportion 80/20. Fraction C was purified by low-pressure chromatography using a binary mixture made up with methylene chloride and methanol in the proportion 90:10 to give 1 (10 mg). Fraction D was purified using the same chromatographic conditions to give 2 (11.5 mg). Structural determination of the isolated compounds was carried out by spectrophotometric methods (ID and 2D NMR, mass and UV spectrometry).
Biological assays
Antiplasmodial activity
Quantitative assessment of in vitro. antimalarial activity against the Kl resistant strain was determined by means of the microculture radioisotope technique based on the method previously described by Desjardins et al. (Citation1979) and modified by Ridley et al. (Citation1996). The assay uses the uptake of [3H]hypoxanthine by parasites as an indicator of viability. Continuous in vitro. cultures of asexual erythrocytic stages of Plasmodium falciparum. were maintained following the methods of Trager and Jensen (Citation1976). Compounds were tested against Kl strain (multi-drug pyrimethamine/chloroquine resistant strain) (Thaithong & Beale, Citation1981). Initial concentration of each compound was 30 μg/ml diluted with two-fold dilutions to make seven concentrations, the lowest being 0.47 μg/ml. After 48 h incubation of the parasites with the compound at 37°C, [3H]hypoxanthine (Amersham 115 Int., Buckinghamshire, UK) was added to each well and the incubation was continued for another 24 h at the same temperature. IC50 was calculated by linear interpolation between the two drug concentrations above and below 50% (Huber & Koella, Citation1993). Chloroquine and artemisinin were used as positive references. The values are means of two independent assays. Each assay was run in duplicate.
Antitrypanosomal and leishmanicidal assays
Trypanosoma brucei rhodesiense The assays were performed according to the procedures described by Freiburghaus et al. (Citation1996). The compounds were dissolved in 10% DMSO, and working stock solutions of 1 mg/mL in serum containing culture medium were prepared. Diluted compounds (100 μL) were pipetted in duplicate into the first row of a 96-well microtiter plate (Costar, Corning, NY, USA). With complete culture medium, three-fold serial dilutions were prepared. After the addition of Trypanosoma brucei rhodesiense. bloodstream-form trypanosomes from axenic culture, the concentrations of the compounds ranged from 500 to 0.07 μg/mL. The total number of trypanosomes in each well was 2 × 102/100 μL. The plate was then incubated for 72 h at 37°C in 5% CO2. Two hours before the end of the incubation 10 μL of Alamar blue solution was added. Fluorescence was measured after 2 h of incubation with the dye Alamar blue in a fluorescence plate reader at 530-nm excitation and 590-nm emission wavelength (Cytofluor 2300, Millipore, Bedford, MA, USA) (Räz et al., Citation1997). IC50 values were calculated from the sigmoidal inhibition curve. The values are means of two independent assays. Each assay was run in duplicate.
Trypanosoma cruzi Rat skeletal myoblasts (L-6 cells) were seeded in 96-well microtiter plates at 2000 cells per well per 100 μL in RPMI 1640 medium with 10% FBS and 2 mM l-glutamine. After 24 h, 5000 trypomastigotes of T. cruzi. were added in each well (100 μL) with or without a serial drug dilution. The plates were incubated at 37°C in 5% CO2 for 4 days. After 96 h, the minimum inhibitory concentration (MIC) was determined microscopically. For measurement of the IC50, the substrate CPRG/Nonidet was added to the wells. The color reaction that developed during the following 2–4 h was read photometrically at 540 min. IC50 values were calculated from the sigmoidal inhibition curve. The values are means of two independent assays. Each assay was run in duplicate.
Leishmanicidal activity Fifty microliters of culture medium, a 1:1 mixture of SM medium (Cunningham, Citation1977) and SDM-79 medium (Bran and Schönenberger, Citation1979) at pH 5.4 supplemented with 10% heat-inactivated FBS, was added to each well of a 96-well microtiter plate (Costar). Serial drag dilutions in duplicates were prepared covering a range from 30 to 0.041 μg/mL. Then, 105 axenically grown L. donovani. amastigotes (strain MHOM/ET/67/L82) in 50 μŁ medium were added to each well and the plate incubated at 37°C under a 5% CO2 atmosphere for 72 h. Ten micro-liters of resazurin solution (12.5 mg resazurin dissolved in 100 mL distilled water) were then added to each well and incubation continued for a further 2–4 h. The plate was then read in a Spectramax Gemini XS microplate fluorometer (Molecular Devices, Sunnyvale, CA, USA) using an excitation wavelength of 536 nm and emission wavelength of 588 nm (Räz et al., Citation1997). Fluorescence development was measured and expressed as percentage of the control. Data were transferred into the graphic programe Softmax Pro (Molecular Devices), which calculated IC5o values. The values are means of two independent assays. Each assay was ran in duplicate.
Results and Discussion
The phytochemical analysis of the aerial parts of Thalia geniculata. led to the isolation of five compounds, identified as sitoindoside I [β-sitosterol-(6-O.-hexadecanoyl)-3-O.-β-d-glucoside] (1), daucosterol β-sitosterol-3-O.-β-d-glucoside) (2), stigmasterol (3), β-sitosterol (4), and geranylfarnesol (5) (see ). All the data were consistent with the literature (Khalil & Idler, Citation1980; Ahmad et al., Citation2003; Wanchai et al., Citation2003; Lendl et al., Citation2005).
Sitoindoside I was isolated previously from Musa paradisiaca.(Ghosal et al., Citation1984), Dracaena draco.(Hernandez et al., Citation2004) and Cremanthodium ellisii.(Wang et al., Citation2004). As far as we know, it is the first time that sitoindoside I is described in the Marantaceae family. The only pharmacological property for sitoindoside I that could be found in the literature is related to an anti-ulcerogenic effect (Ghosal et al., Citation1984). Daucosterol was isolated previously from Zea mays, Arctium lappa., and Caulophyllum thalictroides.. It showed aldose-reductase 205 inhibition activity (Fujita et al., Citation1995; Sanghyun et al., Citation2005).
Stigmasterol and β-sitosterol are common phytosterols that were isolated previously from many botanical species. The latter showed anti-inflammatory, antipyretic, antineoplasic, and antimutagenic properties (Bouic, Citation2002; Kun-Young et al., Citation2003; Park et al., Citation2003) together with antioxidant activity (Moreno, Citation2003). Geranylfarnesol was isolated previously from different species of the Theaceae and Poaceae (Akihisa et al., Citation1999). It has the capacity to induce apoptosis in the cellular NB4, APL, and MDS92 lines (Yaguchi et al., Citation1997). No evidence of antiprotozoal activity could be found in the literature for any of the five isolated compounds.
All five compounds were tested in vitro. against P. falciparum. Kl multidrug-resistant strain, T. brucei rhodesiense. STIB 900 strain, T. cruzi. Tulahuen C4 strain, and L. donovani. MHOM-ET-67/L82 strain. The most interesting activity was obtained with geranylfarnesol (5) with significant antiprotozoal activities against 225 P. falciparum. (IC50 = 12.7 ± 1.1 μM) and L. donovani. (IC50 = 13.2 ± 1.0 μM). These results provide scientific evidence supporting the use of T. geniculata. as an anti-malarial remedy in folk medicine.
Acknowledgments
The authors wish to thank the Agence Universitaire de la Francophonie (AUF) (PCSI no. 6313PS562) and the International Foundation for Science (IFS) for financial support. The authors want also to thank C. Anthaume for assistance in the interpretation of the NMR spectra.
References
- Adjanohoun E J, Adjakidjé V, Ahyi M RA, Akeassi L, Akoegninou A, d'Almeida J, Apovo F, Boukef K, Chadare M, Dramane K, Eyme J, Gassita J N, Gbaguidi N, Goudoté E, Guinko S, Houngnon P, Issa L, Keita A, Kiniffo H V, Koné-Bamba D, Musampa Nseyya A, Saadou M, Sodogandji T, de Souza S, Tchabi A, Zinsou Dossa C, Zohoun T. Médecine tradition-nelle et pharmacopée. contribution aux études ethnobotaniques et floristiques en république du bénin. Agence de Cooperation Culturelle et Technique, Paris 1989; 111, 339–405
- Ahmad B, Jan Q, Shumaila B, Iqbal M, Nisar M. Phytochemical evaluation of Chenopodium murale. Linn. Asian J Plant Sci 2003; 2: 1072–1078
- Akihisa T, Koike K, Kimura Y, Sashida N, Matsumoto T, Ukiya M, Nikaido T. Acyclic and incompletely cyclized triterpene alcohols in the seed oils of Theaceae. and Gramineae. Lipids 1999; 34: 1151–1157
- Bouic P JD. Sterols and sterolins: New drugs for the immune system. Drug Discovery Today 2002; 7: 775–778
- Brun R, Schönenberger M. Cultivation and in vitro. cloning or procyclic culture forms of Trypanosoma brucei. in a semi-defined medium. Acta Trop 1979; 36: 289–292
- Cunningham I. New culture medium for maintenance of tsetse tissues and growth of trypanosomatids. J Protozool 1977; 24: 325–329
- Desjardins R E, Canfield C J, Haynes J D, Chilay J D. Quantitative assessment of antimalarial activity in vitro. was determined by a semi-automated microdilution technique. Antimicrob Agents Chemother 1979; 16: 710–718
- Freiburghaus F, Kaminsky R, Nkunya M HH, Brun R. Evaluation of African medicinal plants for their in vitro. trypanocidal activity. J Ethnopharmacol 1996; 55: 1–11
- Fujita T, Ohira K, Miyatake K, Nakano Y, Nakayama M. Inhibitory effect of perillosides A and C, related monoterpene glucosides on aldose reductase and their structure-activity relationships. Chem Pharm Bull 1995; 43: 920–926
- Ghosal S, Kulwant S. Sitoindosides I and II, two new antiulcerogenic sterylacylglucosides from Musa paradisiaca. J Chem Res Synop 1984; 4: 110
- Hernandez J C, Leon F, Quintana J, Estevez F, Bermejo J. Icogenin, a new cytotoxic steroidal saponin isolated from Dracaena draco. Bioorg Med Chem 2004; 12: 4423–4429
- Huber W, Koella J C. A comparison of the three methods of estimating EC50 in studies of drug resistance of malaria parasite. Acta Trop 1993; 55: 257–261
- Khalil M V, Idler D R, Patterson G W. Sterols of scallop III. Characterization of some C-24 epimeric sterol by high resolution nuclear magnetic resonance spectroscopy. Lipids 1980; 15: 69–73
- Kun-Young P, Jung K O, Rheea S H, Yung H C. Antimutagenic effects of doenjang. (Korean fermented soypaste) and its active compounds. Mutat Res 2003; 523–524: 43–53
- Lendl A, Werner I, Glasl S, Christa K, Mucaji P, Presser A, Reznicek G, Jurenitsch J, Taylor D W. Phenolic and terpenoid compounds from Chione venosa. (SW) Urban var. venosa. Phytochemistry 2005; 66: 2381–2387
- Moreno J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic Biol Med 2003; 35: 1073–1081
- Park D S, Choi S Z, Kim K R, Lee S M, Lee K R, Pyo S. Immunomodulatory activity of triterpenes and phenolic compounds from Viscum album. L. Appl Pharmacol 2003; 11: 1–4
- Räz B, Iten M, Grether Y, Kaminsky R, Brun R. The Alamar Blue® assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense. and T. b. gambiense in vitro. Acta Trop 1997; 68: 139–147
- Ridley R G, Werner H, Hugues M, Catherine J, Arnulf D, Raffaello M, Synese J, Wolfgang F R, Alberto G, Maria A G, Heinrich U, Werner H, Sodsri T, Wallace P. 4-Aminoquinoleme analogs of chloroquine with shor tened side chains retain activity against chloroquine-resistant Plasmodium falciparum. Antimicrob Agents Chemother 1996; 40: 1846–1854
- Sanghyun L, Shim S K, Sun J, Hyun S K, Kang S S. Aldose reductase inhibitors from the fruiting bodies of Ganoderma applanatum. Biol Pharm Bull 2005; 28: 1103–1105
- Thaithong S, Beale G H. Resistance of 10 Thai isolates of Plasmodium falciparum. to chloroquine and pyrimethamine by in vitro. tests. Trans R Soc Trop Med Hyg 1981; 75: 271–273
- Trager W, Jensen J B. Human malaria parasites in continuous culture. Science 1976; 193: 673–675
- Wanchai D-E, Bupparchart P. Biosynthesis of β-sitosterol and stigmasterol in Croton sublyratus. proceeds via a mixed origin of isoprene units. Phytochemistry 2003; 62: 389–398
- Wang A-X, Qi Z, Jia Z-J. Phenylpropanosids, lignans and other constituents from Cremanthodium ellisii. Pharmazie 2004; 11: 889–892
- Wellems T, Plowe C. Chloroquine-resistant malaria. J Infect Dis 2001; 184: 770–776
- Weniger B, Lagnika L, Vonthron-Sénécheau C, Adjobimey T, Gbenou J, Moudachirou M, Brun R, Anton R, Sanni A. Evaluation of ethnobotanically selected Benin medicinal plants for their in vitro. antiplasmodial activity. J Ethnopharmacol 2004; 90: 279–284
- Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, Toyama K. Vitamin K2 and its deri-vatives induce apoptosis in leukemia cells and enhance the effect of all-trans. retinoic acid. Leukemia 1997; 11: 779–787