Abstract
The antidiabetic, hypolipidemic, and antioxidant properties of Phyllanthus niruri. (L) (Euphorbiaceae) were compared in normal, insulin-dependent diabetes mellitus (IDDM), and non–insulin-dependent diabetes mellitus (NIDDM) animals through evaluating the effects on carbohydrate and lipid metabolism and antioxidant activities. The alcohol extract of Phyllanthus niruri. produced significant antidiabetic effect in IDDM alone but lowered lipid profiles and improved body antioxidant activities in both IDDM and NIDDM animals. This investigation revealed that the lipid-lowering effect of Phyllanthus niruri. is independent from its antidiabetic action.
Introduction
Diabetes mellitus (DM) is one of the major health problems affecting a significant portion of the population worldwide (Zimmet et al., Citation2001). DM is classified as insulin-dependent diabetes mellitus (IDDM) and non–insulin-dependent diabetes mellitus (NIDDM). Both IDDM and NIDDM share most common symptoms such as long-term hyperglycemia leading to macrovascular and microvascular complications (Attele et al., Citation2002). It has been established that hyperglycemia is the principal cause of diabetic complications (Brownlee Citation2001). Although glucose levels are controlled by medication, atherosclerosis can still develop in diabetic patients (Steinberg et al., Citation2000), which is a potent independent risk factor for cardiovascular disease (CVD) (American Diabetes Association, Citation1998). People with diabetes exhibit a pattern of dyslipidemia characterized by elevated triglycerides and low levels of high-density lipoprotein cholesterol (HDL-C) (Resnick et al., Citation2002). In addition, diabetic conditions increase oxidative stress (Glugliano et al., Citation1996) and deplete antioxidant levels in the body (Fonseca et al., Citation2004). Therefore, improvement in glucose, lipid, and antioxidant profiles could be useful in management of diabetes and its complications.
Phyllanthus niruri. L. (Euphorbiaceae) (syn. Phyllanthus amarus. Schum & Thonn) is a traditionally well-known plant in India for its Ayurvedic and folklore medicinal uses such as in the treatment of jaundice (Raja Reddy, Citation1988), hepatitis, dysentery, and irritating sores (Reddy et al., Citation1993). The aqueous and methanol extracts of the leaves of this plant were reported to be hypoglycemic (Ramakrishnan et al., Citation1982; Raphael et al., Citation2002), hypotensive and hypoglycemic in human hypertensive (and secondarily diabetic) subjects but was found to be neither hypercholesterolemic nor hypocholesterolemic (Srividya & Periwal, Citation1995). However, Khanna et al. (Citation2002) reported that Phyllanthus niruri. lowers the serum lipid levels in hyperlipemic rats and the antioxidant activity of this plant has been demonstrated in vitro. (Raphael et al., Citation2002). These reports, however, pertain to alloxan-induced diabetic animals, hypertensive human subjects, hyperlipemic animals, and in vitro. observations. Besides, the aqueous extracts of P. amarus. were shown to enhance the clearance of oral glucose load though they did not lower fasting blood glucose (FBG) in normal albino rabbits (Moshi et al., Citation1997) but were ineffective in lowering either FBG or postprandial blood glucose levels in NIDDM subjects (Moshi et al., Citation2001). As no reports are available with reference to comparative studies on the effect(s) of Phyllanthus niruri. on on carbohydrate, lipid metabolism, and antioxidant activity in IDDM and NIDDM subjects, a comparative evaluation of antidiabetic, hypolipidemic, and antioxidant properties of Phyllanthus niruri. in normal, IDDM, and NIDDM animals was undertaken.
Materials and Methods
Collection and extract preparation
The young leaves of the plant Phyllanthus niruri. L. were collected from the university's botanical garden and authenticated by Dr. A.S. Reddy, Department of Biosciences, Sardar Patel University (voucher specimen no. JHB-01). Shade-dried leaves were powdered and extracted with 95% ethanol. The extract (PnlE) was filtered, dried at room temperature (yield 13.3% w/w), and the residue was stored at 4°C for later use.
Animals
Male Charles Foster rats were used for the current study. Animals were housed in polypropylene cages and maintained under ambient room temperature. They were fed standard pellet diet (Pranav Agro Ltd., Pune, India) and water ad libitum.. The institutional animal ethics committee approved the study.
Induction of diabetes
Insulin-dependent diabetes mellitus
Rats weighing 200–250 g were fasted overnight and then given a single intraperitoneal (i.p.) injection of alloxan monohydrate (120 mg/kg b.w., Loba Chemie, Mumbai, India) dissolved in normal saline. Induction of diabetic condition was confirmed by FBG estimation (>140 mg/dL) over a period of 2 weeks.
Non–insulin-dependent diabetes mellitus
The model was developed according to the description of Bonner-Weir et al. (Citation1981). Males aged 48 ± 2 h were injected (i.p.) with streptozotocin (Sisco Research Laboratories, Mumbai, India) in citrate buffer (pH 4.5) at a dose of 100 mg/kg b.w. The animals showing FBG levels >140 mg/dL after 12 weeks were considered as diabetic animals.
Experimental procedure
For the experiment, 48 rats were used (16 normal, 16 IDDM, and 16 NIDDM). Six groups of eight animals each were divided as follows. Group I: normal, vehicle-administered animals; group II: normal, PnlE-administered animals; group III: IDDM, vehicle-administered animals; group IV: IDDM, PnlE-administered animals; group V: NIDDM, vehicle-administered animals; and group VI: NIDDM, PnlE-administered animals.
Treatment was given at a dose of 300 mg/kg b.w. per day in 1 mL of 2% (v/v) Tween 80 suspension for 4 weeks by oral route. Control animals received equal volume of vehicle (1 mL of 2% Tween 80) only. At the end of the experimental period, the animals were deprived of food overnight and sacrificed under mild anesthesia; blood and liver tissues were collected and analyzed immediately.
Oral glucose tolerance test
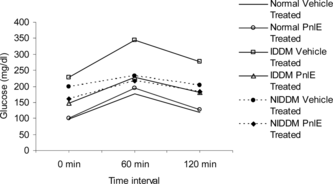
Two days before the termination of the experiment, the oral glucose tolerance test (OGTT) was performed to assess the sensitivity to high glucose load. For this purpose, overnight-fasted rats were fed orally 2 g glucose/kg b.w. Blood was collected at 0-, 60-, and 120-min intervals from orbital sinus for glucose estimation.
Analytical methods
Plasma glucose levels were measured by the o.-toluidine method (Webster et al., Citation1971), hepatic glycogen was extracted with 30% KOH, and the yield was determined by anthrone-sulfuric acid method (Seifter et al., Citation1950). Plasma cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) were estimated by ferric perchlorate-sulfuric acid and glycero-3-phosphate: O2 2-oxidoreductase (GPO) methods, respectively (Wybenga et al., Citation1970; McGown et al., Citation1983). Low-density lipoprotein cholesterol (LDL-C), very-low-density lipoprotein cholesterol (VLDL-C), and atherogenic index (AI) were calculated (Friedwald et al., Citation1972). The hepatic hexokinase (EC 2.7.1.1) was determined following the methods prescribed by Brandstup et al. (Citation1957). The hepatic lipids were extracted (Folch et al., Citation1957) and used for the estimation of total cholesterol and triglyceride content (Wybenga et al., Citation1970; McGown et al., Citation1983). The peroxidation product, thiobarbituric acid reactive substances (TBARS), was determined by the method of Niehaus & Samuelsson (Citation1968). Activities of antioxidant enzymes superoxide dismutase (EC 1.15.1.1) and catalase (EC 1.11.1.6) were estimated according to the methods described by Kakkar et al. (Citation1984) and Aebi (Citation1974), respectively.
Statistical analysis
Data are presented as mean±SEM. One-way analysis of variance (ANOVA) with Tukey's significant difference post hoc test was used to compare differences among groups. Data were statistically handled by SPSS statistical software, version 10. p values <0.05 were considered as statistically significant.
Results
Both IDDM and NIDDM control animals registered significantly higher plasma glucose levels (p < 0.001). A significant reduction in plasma glucose level was noted only in PnlE-administered IDDM animals (p < 0.001). However, both IDDM and NIDDM animals registered significant reduction in TC, TG, LDL-C, VLDL-C, and AI and increases in HDL-C levels with PnlE administration when compared with vehicle-administered control groups (Tables and ).
Table 1.. Effect of PnlE on various biochemical parameters in IDDM rats.
Table 2.. Effect of PnlE on various biochemical parameters in NIDDM rats.
The hepatic glycogen content of IDDM and NIDDM control animals declined significantly (p < 0.001). The IDDM animals alone registered a significant rise in hepatic glycogen level (p < 0.001) with PnlE administration although both NIDDM and normal groups revealed a small increase in hepatic glycogen content upon PnlE administration. The PnlE treatment to IDDM and NIDDM animals caused a significant decline in hepatic TC and TG levels (Tables and ).
Both IDDM and NIDDM controls registered significant decline in hexokinase activity (p < 0.001; p < 0.01). The hexokinase activity increased significantly in the PnlE-administered IDDM group alone (p < 0.001) (Tables and ).
The PnlE treatment to IDDM and NIDDM animals decreased TBARS significantly (p < 0.01, p < 0.05, respectively) and caused a significant increase in catalase activity (p < 0.01, p < 0.05, respectively). However, SOD activity remained more or less unaltered in these groups (Tables and ).
A marked reduction in plasma glucose level occurred in the PnlE-treated IDDM group at 120 min compared with its counterpart. However, no improvements were found in NIDDM and normal animals ().
Discussion
In the current study, a significant increase in fasting blood glucose and decreases in hepatic glycogen levels and hexokinase activity was found in both untreated IDDM and NIDDM animals. PnlE administration caused a significant reduction in plasma glucose levels, increased hepatic glycogen content, and heightened the hepatic hexokinase activity in IDDM animals alone. Further, these animals also exhibited a marked improvement in glucose tolerance. The pronounced hypoglycemic effect of PnlE in IDDM animals could be due to either its insulin-like activity or its stimulatory effect on insulin. On the other hand, the lack of hypoglycemic activity of PnlE in NIDDM animals could be due to its inability to decrease the insulin resistance in these animals (Kergoat & Portha, Citation1985; Santosh et al., Citation2003). This lack of hypoglycemic activity of PnlE in NIDDM animals is reflected in their poor glucose tolerance.
Both IDDM and NIDDM control animals exhibited marked increases in plasma and hepatic lipid profiles along with decreased HDL-C levels. Diabetes is associated with increased plasma and tissue cholesterol and triglyceride levels with lowered HDL-C profile (Glasgow et al., Citation1981; Goodman et al., Citation1982; Kudchodkar et al., Citation1988). Currently, PnlE administration to both IDDM and NIDDM animals caused a significant decline in both serum and hepatic lipid levels and increased the HDL-C levels. The significant decreases in LDL-C and increases in HDL-C levels in PnlE-administered IDDM and NIDDM animals on the other hand indicate the altered lipid metabolism under the influence of PnlE that are similar to the reported decline in LDL-C and elevation of HDL-C profiles in hyperlipemic rats treated with Phyllanthus niruri. extract (Khanna et al., Citation2002). Consequent to lowered plasma lipid profiles, both PnlE-administered IDDM and NIDDM animals registered a significant decrease in atherogenic index. Thus, while the lipid-lowering activity of PnlE appeared to be common to both IDDM and NIDDM animals, the hypoglycemic activity of PnlE is restricted to the IDDM animals alone even with a 4-week administration of PnlE (300 mg/kg b. w. per day). In this context, it is pertinent to note here that a 1-week treatment with P. amarus. extract (12.5 g% dose) could not lower both FBG and postprandial blood glucose in NIDDM patients (Moshi et al., Citation2001) although a similar dose of extract (0.1 and 1.0 g/kg b. w.) could enhance the clearance rate of oral glucose load from blood without significantly affecting FBG levels in rabbits (Moshi et al., Citation1997). The stem bark extract of a related species of the genus Phyllanthus. (i.e., P. sellowianus.), however, was found to significantly reduce the blood glucose level in streptozotocin-induced diabetic (IDDM) mice at a dose of 200 mg/kg b. w. (Hnatyszyn et al., Citation2002). It is therefore evident that P. niruri. (P. amarus.) is ineffective in NIDDM with reference to maintenance of plasma glucose levels.
Chronic hyperglycemia induces lipid peroxidation, overproduction of superoxides, and produces oxidative stress (Nakakimura & Mizuno, Citation1980; Baynes Citation1991; Glugliano et al., Citation1996; Maxwell et al., Citation1997). Both IDDM and NIDDM groups registered increased TBARS, a marker of lipid peroxidation (24% increases over controls), and the PnlE administration significantly decreased the level of TBARS in these groups. SOD and catalase are known to be important antioxidant enzymes that catalyze superoxide radicals and reduce peroxides, respectively (Eriksson & Borg, Citation1991). Although the induction of diabetes significantly reduced the hepatic catalase activity in both diabetic groups, the SOD activity declined only marginally. Oral administration of PnlE to both IDDM and NIDDM animals significantly increased the catalase activity accompanied by a small increase in SOD activity. The activities of these enzymes in normal animals on the other hand did not register any changes upon PnlE administration.
Thus, our study shows that PnlE is both hypoglycemic and hypolipidemic in IDDM animals and predominately hypolipidemic in NIDDM animals. However, PnlE exhibited its antiperoxidative and antioxidant activities in both experimental diabetic groups. The effects of PnlE on normal animals were neither hypoglycemic nor hypolipidemic but appeared to maintain the euglycemic and eulipidemic status of these animals. Further investigation in a dose-dependent manner is in progress.
Acknowledgment
The financial assistance in the form of a research fellowship to J.H.B. from the Puri Foundation, Gandhinagar, Gujarat, India, is gratefully acknowledged.
References
- Aebi H (1974): Catalase. In: Bergmeyer HU, ed., Methods of Enzymatic Analysis. New York, Academic Press, pp. 673–684.
- American Diabetes Association (1998): Economic consequences of diabetes mellitus in the US in 1997. Diabetes Care 21: 296–309.
- Attele AS, Zhou YP, Xia JT, Wu JN, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS (2002): Antidiabetic effects of Panax ginseng. berry extract and the identification of an effective component. Diabetes 51: 1851–1858.
- Baynes JW (1991): Role of oxidative stress in development of complications in diabetes. Diabetes 40: 405–412.
- Bonner-Weir S, Trent DF, Honey RN, Weir GC (1981): Response of neonatal rat islets to streptozotocin. Limited β cell regeneration and hyperglycemia. Diabetes 30: 64–69.
- Brandstup N, Kirk JE, Bruni C (1957): Determination of hexokinase in tissues. J Gerontol 12: 166–171.
- Brownlee M (2001): Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820.
- Eriksson UJ, Borg LAH (1991): Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformation in vitro.. Diabetologia 34: 325–331.
- Folch J, Lees MS, Stanley GH (1957): A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509.
- Fonseca V, Desouza C, Asnani S, Jialal I (2004): Nontraditional risk factors for cardiovascular disease in diabetes. Endocr Rev 25(1): 153–175.
- Friedwald WT, Levy RT, Fredrickson DS (1972): Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502.
- Glasgow AM, August GP, Hung W (1981): Relationship between control and serum lipids in juvenile onset diabetes. Diabetes Care 4: 76–80.
- Glugliano D, Ceriello A, Paolissa G (1996): Oxidative stress and diabetic vascular complications. Diabetes Care 19: 257–267.
- Goodman MW, Michels LD, Keane WF (1982): Intestinal and hepatic cholesterol synthesis in the alloxan diabetic rats. Proc Soc Exp Biol Med 170: 286–290.
- Hnatyszyn O, Mino J, Ferraro G, Acevedo C (2002): The hypoglycemic effect of Phyllanthus sellowianus. fractions in streptozotocin induced diabetic mice. Phytomedicine 9: 556–559.
- Kakkar P, Das B, Viswanathan PN (1984): A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21: 130–132.
- Kergoat M, Portha B (1985): In vivo. hepatic and peripheral insulin sensitivity in rats with non-insulin-dependent diabetes induced by streptozotocin. Assessment with the insulin-glucose clamp technique. Diabetes 34: 1120–1126.
- Khanna AK, Rizvi F, Chander R (2002): Lipid lowering activity of Phyllanthus niruri. in hyperlipaemic rats. J Ethnopharmacol 82: 19–22.
- Kudchodkar BJ, Lee JC, Lee SM, DiMareo NM, Lacko AG (1988): Effect on cholesterol homeostasis in diabetic rats. J Lipid Res 29: 1272–1287.
- Maxwell SRJ, Thomson H, Sandler D, LeGuen C (1997): Poor glycaemic control is associated with reduced serum free radical scavenging (antioxidant) activity in non-insulin dependent diabetes mellitus. Ann Clin Biochem 34: 638–644.
- McGown MW, Artiss JD, Strandberg DR, Zak B (1983): A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29: 538–542.
- Moshi MJ, Lutale JJK, Rimoy GH, Abbas ZG, Josiah RM, Swai ABM (2001): The effect of Phyllanthus amarus. aqueous extract on blood glucose in non-insulin dependent diabetic patients. Phytother Res 15: 577–580.
- Moshi MJ, Uiso FC, Mahunnah RLA, Malele SR, Swai ABM (1997): A study of the effect of Phyllanthus amarus. extracts on blood glucose in rabbits. Pharm Biol 35: 167–173.
- Nakakimura H, Mizuno K (1980): Studies on lipid peroxidation in biological systems II. Hyperlipoperoxidemia in mice induced by alloxan. Chem Pharm Bull 28: 2207–2211.
- Niehaus WG Jr, Samuelsson B (1968): Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6: 126–130.
- Raja Reddy K (1988): Folk medicine from Chittoor District, Andhra Pardesh, India, used in the treatment of jaundice. Int J Crude Drug Res 26: 127–140.
- Ramakrishnan PN, Murugan R, Palanichamy S, Murugesh N (1982): Oral hypoglycemic effect of Phyllanthus niruri.. Indian J Pharm Sci 44: 10–12.
- Raphael KR, Sabu MC, Kuttan R (2002): Hypoglycemic effect of methanolic extract of Phyllanthus amarus. Schum & Thonn on alloxan induced diabetes mellitus in rats and its relation with antioxidant potential. Indian J Exp Biol 40: 905–909.
- Reddy MB, Bryan HH, Lance CJ, McMillan RT Jr (1993): A survey of plant crude drugs of Anantapur district, Andhra Pradesh, India. Econ Bot 47: 79–88.
- Resnick HE, Howard BV (2002): Diabetes and cardiovascular disease. Annu Rev Med 53: 245–267.
- Santosh LV, Rajani M, Goyal RK (2003): Comparative antidiabetic activity of different fractions of Enicostemma littorale. Blume in streptozotocin induced NIDDM rats. Orient Pharm Exp Med 3(4): 196–204.
- Seifter S, Dayton S, Noyic B, Muntwyler E (1950): Estimation of glycogen with anthrone reagent. Arch Biochem 25: 191–200.
- Srividya N, Periwal S (1995): Diuretic, hypotensive and hypoglycaemic effects of Phyllanthus amarus.. Indian J Exp Biol 33: 861–864.
- Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD (2000): Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49: 1231–1238.
- Webster WW, Stinson SF, Wong WH (1971): Manual procedure for direct microassay of serum glucose by o.-toluidine, and its adaptation to the SMA 12/60 autoanalyser. Clin Chem 17: 1050–1054.
- Wybenga DR, Pileggi VJ, Dirstine PH, Di Giorgio J (1970): Direct manual determination of serum total cholesterol with a single stable reagent. Clin Chem 16: 980–984.
- Zimmet P, Alberti KGMM, Shaw J (2001): Global and societal implications of the diabetes epidemic. Nature 414: 782–787.