Abstract
4-Hydroxy-4, 7-dimethyl-α.-tetralone (1), 4,5-dihydroblumenol A (2), N.-trans.-feruloyltyramine (3), and 24–methylenelanosta-7, 9(11)-dien-3-β., 15α.-diol (4) were isolated from the stem bark of Polyalthia jucunda. (Piere) Finet & Gagnep (Annonaceae). All the compounds were evaluated for their effects on growth of four human tumor cell lines [ER(+) MCF-7, ER(−) MDA-MB-23, SF 268, and NCI-H460] and of a non–tumor cell line (MRC-5). Only compound 4 exhibited a dose-dependent growth inhibitory effect against both tumor and non–tumor cell lines but with less effect on the latter. Using the TUNEL assay, it was found that the inhibitory effect of compound 4 on NCI-H460 cells was probably caused by apoptosis.
Introduction
The genus Polyalthia. (Annonaceae) comprises 120 species of shrubs and trees, which are common to tropical and subtropical regions from Africa to Southeast Asia (Connolly et al., Citation1996). The plants of this genus have been found to afford a variety of secondary metabolites, including aporphine (Hamonnière et al., Citation1977; Jossang et al., Citation1984; Yang-Chang et al., Citation1990; Connolly et al., Citation1996; Kanokmedhakul et al., Citation2003), azaanthracene (Tuchinda et al., Citation2000), azafluorene (Yang-Chang et al., Citation1990), indolosesquiterpene (Hamonnière et al., Citation1977; Hocquemiller et al., Citation1981; Okorie, Citation1981) and protoberberine (Gonzalez et al., Citation1997, Chung-Yi et al., Citation2000; Faizi et al., Citation2003) alkaloids; benzopyran derivatives (Zafra-Polo et al., Citation1996); clerodane (Kijjoa et al.,Citation1989Citation1990Citation1993; Ma et al., Citation1994; Hao et al., Citation1995; Hara et al., Citation1995), halimane (Hara et al., Citation1995; Chung-Yi et al., Citation2000), and labdane diterpenes (Richomme et al., Citation1991); diynoic acids (Kanokmedhakul et al., Citation1998; Tuchinda et al., Citation2001); N.-cinnamoyltyramine (Tuchinda et al., Citation2001) and lanostane-type triterpenes (Li et al., Citation1993; Lue et al., Citation1998), and some of these compounds have been found to exhibit interesting biological activity. Whereas some of the aporphine alkaloids and clerodane diterpenes exhibited a cytotoxic effect on human cancer cell lines (Yang-Chang et al., Citation1990; Ma et al., Citation1994), the labdane diterpene was found to possess leishmanicidal activity (Richomme et al., Citation1991). Interestingly, the C31 lanostane-type triterpene suberosol and the azaanthracene alkaloid kalasinamide, isolated from Polyalthia suberosa. (Roxb.) Thw., were shown to exhibit anti-HIV replication activity (Li et al., Citation1993; Tuchinda et al., Citation2001). On the other hand, the dimeric aporphine alkaloids bidebilines C and D, constituents of the roots of Polyalthia debilis. (Piere) Finet & Gagnep, were found to exhibit a moderate antimalarial activity against a multidrug-resistant strain of Plasmodium falciparum. (Kanokmedhakul et al., Citation2003).
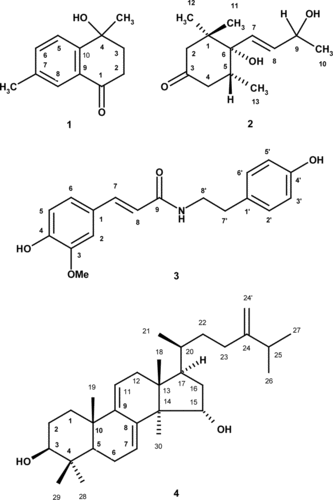
During our search for bioactive constituents from Thai plants, we have investigated the constituents of Polyalthia jucunda. (Piere) Finet & Gagnep, collected from the north east of Thailand. Besides 4-hydroxy-4, 7-dimethyl-α.-tetralone (1) and 4,5-dihydroblumenol A (2), N.-trans.-feruloyltyramine (3) and 24–methylenelanosta-7, 9(11)-dien-3-β., 15α.-diol (4) have been isolated from its stem bark (). The structure of the compounds was established by spectral analysis (1H, 13C, COSY, HSQC, HMBC, and NOESY) as well as HRMS. All the isolated compounds were tested for their in vitro. effect on growth of four human tumor cell lines [ER(+) MCF-7, ER(−) MDA-MB-23, SF 268 and NCI-H460] and of a non–tumor cell line (MRC-5).
Materials and Methods
General experimental procedure
1H and 13C NMR were recorded at ambient temperature in CDCl3, with a Bruker AMC instrument (Wissenbourg, France) operating at 300.13 and 75.74 MHz. Electron Impact (EI) mass spectra were measured on a Hitachi Perkin-Elmer RMV-6M instrument (Perkins-Elmer, USA). High Resolution Mass Spectra (HRMS) were measured on a Kratos Concept II 2 sector/mass spectrometer (Kratos Analytical, Urmston, Manchester, UK). Rotations were determined on a Polax-2 L instrument (Atago Co. Ltd., Tokyo, Japan). Silica gel 60 (0.063–0.200 mm; Merck, Damstadt, Germany) for column chromatography and Si gel 60 GF254 (Merck, Damstadt, Germany) for analytical and preparative TLC were used.
Plant material
Polyalthia jucunda. (Pierre) Finet & Gagnep (Annonaceae) was collected at Khao Yai National Park, Nakhorn Ratchasima, Thailand, in November 2004. The plant material was identified by comparison with the herbarium specimen (voucher no. 121063) of the Royal Forest Department, Bangkok, Thailand.
Extraction and isolation
Dried and powdered stem bark of Polyalthia jucunda. (3.5 kg) was percolated by hexane (5 × 4 L) at room temperature. The hexane solution was evaporated under reduced pressure to give a hexane extract (16 g). The residue was percolated by dichloromethane (5 × 4 L) at room temperature, and the CHCl2 solution was evaporated under reduced pressure to give a crude dichloromethane extract (22.6 g). The residue was then percolated by methanol to exhaustion (5 × 4 L) at room temperature, and the methanol solution was evaporated under reduced pressure to give a crude methanol extract (165 g), which was then dissolved in ethyl acetate (5 × 4 L). The ethyl acetate solutions were combined and evaporated under reduced pressure to give an ethyl acetate extract (8.3 g).
The dichloromethane extract (22.6 g) was applied to a Si gel column (300 g) and eluted with petrol-CHCl2, CHCl2, CHCl2-MeOH, 250-mL fractions being collected as follows: Fractions 1–22 (petrol-CHCl2, 1:1), 23–83 (petrol-CHCl2, 3:7), 84–112 (petrol-CHCl2, 1:9), 113–143 (CHCl2), 144–189 (CHCl2-MeOH, 9.5:0.5). Fractions 25–31 (550 mg) on combination and recrystallization from methanol furnished β.-sitosterol (205 mg). Fractions 113–143 were combined (420 g) and applied to a Si gel column (20 g) and eluted with petrol-CHCl2, CHCl2, CHCl2-MeOH, 20-mL subfractions being collected as follows: Subfractions 1–28 (petrol-CHCl2, 4:1), 29–50 (petrol-CHCl2, 7:3), 51–62 (CHCl2), 63–80 (CHCl2-MeOH, 95:5). Subfractions 54–62 were combined (62 mg) and purified by TLC (Si gel, CHCl2-Me2O, 98:2) to give 4-hydroxy-4,7-dimethyl-α.-tetralone (1, 15 mg).
The ethyl acetate extract (8.3 g) was applied to a Si gel column (250 g) and eluted with CHCl2, CHCl2-Me2O, Me2O, 200-mL fractions being collected as follows: Fractions 1–36 (CHCl2), 37–62 (CHCl2-Me2O, 95:5), 63–72 (CHCl2-Me2O, 9:1), 73–93 (CHCl2-Me2O, 7:3), 94–110 (CHCl2-Me2O, 1:1), 111–139 (CHCl2-Me2O, 3:7), 140–156 (CHCl2-Me2O, 1:9). Fractions 73–81 were combined (110 mg) and applied to a Si gel column (20 g) and elution with a mixture of CHCl2-Me2O (9:1) gave 12 subfractions, 20-mL each. Subfractions 3–12 were combined (80 mg) and purified by TLC (Si gel, CHCl2-Me2O, 9:1) to give 4,5-dihydroblumenol A (2, 6.5 mg) and N.-trans.-feruloyltyramine (3, 11.5 mg).
Fractions 140–147 were combined (110 mg) and purified by TLC (Si gel, petrol-CHCl2, 98:2) to give white crystals of 24–methylenelanosta-7, 9(11)-dien-3-β., 15α.-diol (4, 20 mg).
4-Hydroxy-4,7-dimethyl-α-tetralone (1)
1H NMR (CDCl3, 300 MHz): 7.81d (J. = 2.0, H-8), 7.60d (J. = 8.0, H-5), 7.43d (J. = 8.0, 2.0, H-6), 2.88 ddd (J. = 17.8, 5.2, 5.2, H-2a), 2.69 ddd (J. = 17.8, 9.2, 7.1, H-2b), 2.28d (J. = 5.3, H-3a), 2.26 dd (J. = 5.3, 2.0, H-3b), 2.28s (Me-7), 1.63s (Me-4). 13C NMR (CDCl3, 75.47 MHz): 197.6 (C-1), 146.6 (C-10), 137.8 (C-7), 135.2 (C-6), 130.3 (C-9), 127.2 (C-8), 125.2 (C-5), 70.2 (C-4), 38.4 (C-3), 35.9 (C-2), 29.0 (Me-4), 21.0 (Me-7). EI-MS: m/z. (%), 190 (M+, 48), 175 (100), 162 (39), 119 (30). = + 100.2°(CDCl3, c = 0.02 g/100 mL).
4,5-Dihydroblumenol A (2)
1H NMR (CDCl3, 300 MHz): 5.84 dd (J. = 15.7, 5.6, H-8), 5.70 dd (J. = 15.7, 1.0, H-7), 4.44q (J. = 5.7, H-9), 2.84d (J. = 13.6, H-2a), 2.42t (J. = 12.1, H-4a), 2.15–2.25m (H4b), 1.92 dd (J. = 13.6, 2.1, H-2b), 1.33d (J. = 6.4, Me-10), 0.97s (Me-12), 0.94s (Me-11), 0.88d (J. = 6.4, Me-13). 13C NMR (CDCl3, 75.47 MHz): 211.4 (C-3), 135.1 (C-8), 131.8 (C-7), 68.3 (C-9), 77.3 (C-6), 51.4 (C-2), 45.1 (C-4), 42.5 (C-1), 36.4 (C-5), 24.5 (Me-11), 24.4 (Me-12), 23.9 (Me-10), 15.9 (Me-13). EI-MS: m/z. (%), 226 (M+, 40), 228 (22), 177 (65), 128 (62), 109 (57), 71 (100). [α.]D24 = + 12.6° (CDCl3, c = 0.33 g/100 mL).
N-trans-Feruloyltyramine (3)
1H NMR (CDCl3, 300 MHz): 9.46 brs (OH), 9.21 brs (OH), 8.00t (J. = 5.6, NH), 7.30d (J. = 15.6, H-7), 7.11 brs (H-2), 7.01d (J. = 8.4, H-2′, H-6′), 6.98d (J. = 8.1, H-6), 6.78d (J. = 8.1, H-5), 6.68d (J. = 8.4, H-3′, 5′), 6.43d (J. = 15.6, H-8), 3.80s (OMe-3), 3.29–3.50 m (H-8′), 2.64 t (J. = 7.1, H-7′). 13C NMR (CDCl3, 75.47 MHz): 165.9 (C-9), 155.7 (C-4′), 148.2 (C-4), 147.8 (C-3), 138.9 (C-7), 129.5 C-1′), 129.5 (C-2′, 6′), 126.4 (C-1), 121.5 (C-6), 119.0 (C-8), 115.6 (C-5), 115.1 (C-3′, 5′), 110.7 (C-2′), 55.5 (OMe-3), 41.0 (C-8′), 34.4 (C-7′). +FAB HRMS: m/z. = 314.13917 (M + H)+; calcd. for C18H20NO4, 314.13925.
24-Methylenelanosta-7, 9(11)-dien-3-β, 15α-diol (4)
1H NMR (CDCl3, 300 MHz): 5.85d (J. = 6.3, H-7), 5.31d (J. = 5.9, H-11), 4.72 s (H-24a), 4.66d (J. = 1.0, H-24b), 4.28 dd (J. = 9.7, 5.2, H-15), 3.25 dd (J. = 10.0, 4.6, J. = 3), 2.30d (J. = 17.2, H-12), 2.06 dd (J. = 17.2, 6.2), 2.21 dd (J = 14.2, 7.2, H-25), 2.05–2.12m (H-23), 1.99 dd (J. = 13.0, 3.1, H-1), 1.88 ddd (J. = 14.7, 10.2, 4.9, H-23), 1.41 dd (J. = 13.0, 4.7, H-1), 1.10d (J. = 4.2, H-5), 1.07d (J. = 4.2, H-17), 1.03d (J. = 6.8, Me-26), 1.02d (J. = 6.9, Me-27), 1.00s (Me-28), 0.95s (Me-30), 0.98s (Me-19), 0.81s (Me-29), 0.61s (Me-18). 13C NMR (CDCl3, 75.47 MHz): 156.5 (C-24), 146.0 (C-9), 140.8 (C-8), 121.28 (C-7), 116.0 (C-11), 106.1 (C-24′), 78.9 (C-3), 74.7 (C-15), 51.9 (C-14), 48.9 (C-5), 48.8 (C-17), 44.3 (C-13), 40.1 (C-16), 38.6 (C-4), 38.5 (C-12), 37.4 (C-10), 36.0 (C-20), 35.7 (C-1), 34.8 (C-22), 33.8 (C-25), 31.2 (C-23), 28.1 (C-28), 27.7 (C-2), 22.9 (C-6), 22.8 (Me-19), 22.0 (Me-26), 21.8 (Me-27), 18.4 (Me-21), 17.1 (Me-30), 15.9 (Me-29), 15.8 (Me-18). +FAB-MS: m/z. (%), 503(M + H)+, 471 (52), 454 (100), 437 (50). = + 56°(CDCl3, c = 0.27 g/100 mL).
Biological activity assays
Tumor cell growth assay
Stock solutions of compounds were prepared in DMSO (Sigma Chemical Co., St. Louis, MO, USA) and stored at − 20°C. The frozen samples were freshly diluted with culture medium just prior to the assays. Final concentrations of DMSO (0.25%) did not interfere with the growth of cell lines. The effects of compounds on the growth of the human tumor and non–tumor cell lines were evaluated according to the procedure adopted by the National Cancer Institute (USA) for the in vitro. anticancer drug discovery screen that uses the protein-binding dye sulforhodamine B (SRB; Sigma Chemical Co.) to assess cell growth inhibition (Skehan et al., Citation1990; Monks et al., Citation1991). Four human tumor cell lines were used, namely the estrogen-dependent ER(+) MCF-7 (breast adenocarcinoma), the estrogen-independent ER(−) MDA-MD-231 (breast adenocarcinoma), NCI-H460 (non–small cell lung cancer), and SF-268 (CNS cancer). The non–tumor cell line MRC-5 (human fetal lung) was also used. NCI-H460 and SF-268 were provided by the National Cancer Institute (NCI, Bethesda, MD, USA); MCF-7, MDA-MB-231, and MRC-5 were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). Cells were routinely maintained as adherent cell cultures in RPMI-1640 medium (Gibco BRL, Scotland, UK) supplemented with 5% heat-inactivated fetal bovine serum (Gibco BRL, Scotland, UK) for MCF-7 and 10% for MDA-MB-231 and MRC-5, 2 mM glutamine (Sigma Chemical Co., St. Louis, MO, USA), 100 U/mL penicillin (Gibco BRL, Scotland, UK), 100 µg/mL streptomycin (Gibco BRL, Scotland, UK), and trypsin (Gibco BRL, Scotland, UK) at 37°C in a humidified atmosphere containing 5% CO2. The optimal plating density of each cell line, which ensures exponential growth throughout all the experimental period, was the same as originally published (Monks et al., Citation1991) and was, respectively 1.5 × 105 cells/mL to MCF-7, MDA-MB-231, MRC-5, and SF-268 and 7.5 × 104 cells/mL to NCI-H460. Cells in 96-well plates were allowed to attach overnight and were then exposed for 48 h to five concentrations of compounds, starting from a maximum concentration of 150 µM. Following this incubation period, the adherent cells were fixed in situ., washed, and dyed with SRB. The bound stain was solubilized and the absorbance was measured at 492 nm in a plate reader (EAR 400, STL-Labinstruments, Linz, Austria). For each compound tested a dose-response curve was generated and the growth inhibition of 50% (GI50), corresponding with the concentration of compound that inhibits 50% of the net cell growth was determined as described (Monks et al., Citation1991). Doxorubicin (Sigma Chemical Co.), used as a positive control, was tested in the same manner.
Apoptosis assay
Fragmentation of the genomic DNA was evaluated using the In situ. Cell Death Detection Kit (TUNEL) Fluorescein (Boehringer, Mannheim, Germany). Exponential NCI-H460 cells growing in 12-well plates were exposed to 40 µM of compound 4 for 24 h. Cells were then washed with PBS (Gibco BRL, Scotland, UK) 5 times for 5 min and fixed with 4% paraformaldehyde (in PBS) for 1 h at room temperature. Fixed cells were washed 3 times for 5 min and permeabilized (0.1% Triton X-100, 0.1% sodium citrate in distilled water) for 2 min on ice (4°C) and washed with PBS 3 times for 5 min. Coverslips were mounted in Vectashield (Vector Laboratories, Peterborough, UK) containing 50 µg/mL propidium iodide (PI) and 0.5 µg/mL RNase. All preparations were observed under the fluorescence microscope Eclipse E400 (Nikon, Japan). Apoptotic cells were quantified by counting a minimum of 400 cells from at least five different random areas of the slide.
Results and Discussion
Though N.-trans.-feruloyltyramine (3) and 24–methylenelanosta-7, 9(11)-dien-3-β., 15α.-diol (4) have been previously isolated from the plants of the genus Polyalthia. (Li et al., Citation1993; Lue et al., Citation1998; Tuchinda et al., Citation2000), 4-hydroxy-4,7-dimethyl-α.-tetralone (1) and 4,5-dihydroblumenol A (2) were isolated for the first time from this genus. While 4,5-dihydroblumenol A (2) was first isolated from the aerial part of Perrottetia multiflora. Lundell (González et al., Citation1994) and later from leaves of Helianthus annuus. var. SH-222® and VYP® (Macías et al., Citation1998), 4-hydroxy-4,7-dimethyl-α.-tetralone (1) was once reported, by a Japanese group, from Hypericum erectum. Thunb. (Matsuoka et al., Citation2004). However, we have no access to the spectroscopic data or to the method of isolation of this compound. Interestingly, except for compound 4, which exhibited an anti-HIV replication activity (Li et al., Citation1993), these compounds have not been investigated for their biological activity.
Compounds 1, 2, 3, and 4 were evaluated for their ability to inhibit the in vitro. growth of four human tumor cell lines, namely, the estrogen-dependent ER(+) MCF-7, the estrogen-independent ER(−) MDA-MB-23, SF-268, and NCI-H460 and of the non–tumor cell line MRC-5. The results are shown in . The results showed that compounds 1, 2, and 3 were ineffective as cell growth inhibitors even when tested at concentrations of 150 µM. On the contrary, compound 4 exhibited, after a continuous exposure of 48 h, an interesting dose-dependent growth inhibitory effect extensive not only against the tumor cell lines but also against the nontumor MRC-5 cells. However, a more pronounced effect was observed against the tumor cells suggesting a high sensitivity of these cells toward this compound.
Table 1.. Effects of compounds 1–4 on the growth of human tumor and non–tumor cell lines.
In order to investigate if compound 4 exerted its growth inhibitory effect by inducing apoptosis, NCI-H460 cells were treated with 40 µM of this compound for 24 h and analyzed for DNA fragmentation by TUNEL assay. Interestingly, the number of apoptotic cells after treatment with compound 4 was significantly higher (23.2%) than that of the nontreated cells (6.84%). This preliminary study indicated that compound 4 might exert its growth inhibitory effect on NCI-H460 cells through the involvement of apoptotic events.
Acknowledgments
This article is dedicated to the memory of Prof. Dr. Vichian Jirawongse, the father of Pharmacognosy in Thailand. Work in Portugal was supported by Fundação para a Ciência e Tecnologia (Unidade de I&D 226/96), POCTI (QA III), FEDER, and CIIMAR Plurianual. We thank Dr. Graham Eaton, Department of Chemistry, Leicester University, UK, for the mass spectra.
References
- Chung-Yi C, Fang-Rong C, Yao-Ching S, Tian-Jye H, Yi-Chen C, Huang-Yi T, Hua-Chien C, Shu-jen C, Ming-Chu H, Yang-Chang W (2000): Cytotoxic constituents of Polyalthia longifolia. var. pendula.. J Nat Prod 63: 1475–1478.
- Connolly J, Hague ME, Kadir AA (1996): Two 7,7′-bisdehydroaporphine alkaloids from Ployalthia bullata.. Phytochemistry 23: 295–295.
- Faizi S, Khan AL, Azher S, Khan SA, Tauseef S, Ahmad A (2003): New antimicrobial alkaloids from the roots of Polyalthia longifolia. var. pendula. Planta Med 69: 350–355.
- González A, Guillermo JA, Ravelo AG, Jimenez IA, Gupta MP (1994): 4,5-Dihydroblumenol, a new nor-isoprenoid from Perrottetia multiflora.. J Nat Prod 57: 400–400.
- Gonzalez MC, Zafra-Polo MC, Bláquez MA, Serrano A, Cortes D (1997): Cerasodine and cerasonine: New oxoprotoberberine alkaloids from Polyalthia cerasoides.. J Nat Prod 60: 108–108.
- Hamonnière M, Leboeuf M, Cave A (1977): Alcalóides aporphiniques et composés terpéniques du Polyalthia oliveri.. Phyochemistry 16: 1029–1029.
- Hao XJ, Yang XS, Zhang Z, Shang LJ (1995): Clerodane diterpenes from Polyalthia. cheliensis.. Phytochemistry 39: 447–447.
- Hara N, Asaki H, Fujimoto Y, Gupta YK, Singh AK, Sahat M (1995): Clerodane and ent-halimane diterpenes from Polyalthia longifolia.. Phyochemistry 38: 189–189.
- Hocquemiller R, Dubois G, Leboeuf M, Cavé M, Kunesch N, Riche C, Chiaroni A (1981): La polyveoline, nouvel indolosesquiterpene isolé du Polyalthia. suaveolens., Annonacees (1). Tetrahedron Lett 22: 5057–5060.
- Jossang A, Leboeuf M, Cave A (1984): Alcaloïdes des Annonacees. L: Alcaloïdes de Polyalthia cauliflora.. J Nat Prod 47: 504–504.
- Kanokmedhakul S, Kanokmedhakul K, Ohtani I, Isobe M (1998): A diynoic acid from Polyalthia evecta. (1998). Phytochemistry 47: 131–133.
- Kanokmedhakul S, Kanokmedhakul K, Yodbuddee D, Phonkerd N (2003): New antimalarial bis.-dehydroaporphine alkaloids from Polyalthia debilis.. J Nat Prod 66: 616–616.
- Kijjoa A, Pinto MMM, Herz M (1989): 16-Hydroxy-3,13Z.-kolavadien-16,15-olide from Polyalthia viridis.. Planta Med 55: 205–205.
- Kijjoa A, Pinto MMM, Pinho PMM, Herz W (1993): Clerodanes from Polyalthia viridis.. Phytochemistry 34: 457–457.
- Kijjoa A, Pinto MMM, Pinho PMM, Tantisewie B, Herz W (1990): Further clerodane derivatives from Polyalthia viridis.. Phytochemistry 29: 653–653.
- Li HY, Sun NJ, Kashiwada Y, Sun L, Snider JV, Cosentino LM, Lee KH (1993): Anti-AIDS agent, 9. suberosol, a new C31 lanostane-type triterpene and anti-HIV principle from Polyalthis suberosa.. J Nat Prod 56: 1130–1130.
- Lue YP, Mu Q, Zheng HL, Li CM (1998): 24-Methylene tetracyclic triterpenes from Polyalthia lancilimba.. Phytochemistry 49: 2053–2053.
- Ma X, Lee LS, Chai HB, Zaw K, Farnsworth NR, Soejarto DD, Cordell GA, Pezzuto JM, Kinghorn AD (1994): Cytotoxic clerodane diterpenes from Polyalthia barnesii.. Phytochemistry 37: 1959–1959.
- Macias FA, Varela RM, Torres A, Oliva RM, Molinillo JM (1998). Bioactive norsesquiterpenes from Helianthus annus. with potential allelopathic activity. Phytochemistry 48: 631–631.
- Matsuoka E, Machida K, Kikuchi M (2004): Studies on the constituents of Hypericum species. I: On the chemical constituents of Hypericum erectum. Thunb. J Tohoku Pharm Univ 51: 41–41.; Chem. Abstr.. 143.: 18989a (2005).
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M (1991): Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83: 757–765.
- Okorie D (1981): Polyavolinamide, an indolosesquiterpene alkaloid from Polyalthia suaveolens.. Phytochemistry 20: 2575–2575.
- Richomme P, Godet MC, Foussard F, Toupet L, Sévenet T, Bruneton J (1991): A novel leishmanicidal labdane from Polyalthia macropoda.. Planta Med 57: 52–52.
- Skehan P, Storeng R, Scudiero D, Monks A, MacMahon J, Vistica D, Warren JT, Bokesch H, Kenny S, Boyd M (1990): New calorimetric cytotoxic assay for anticancer-drug screening. J Natl Cancer Inst 82: 1107–1112.
- Tuchinda P, Pohmakotr M, Munyoo B, Reutrakul V, Santisuk T (2000): An azaantracene alkaloid from Polyalthia suberosa.. Phytochemistry 53: 1079–1079.
- Tuchinda P, Pohmakotr M, Reutrakul V, Thanyachareon W, Sophasan S, Yoosook C, Santisuk T, Pezzuto JM (2001): 2-Substituted furans from Polyalthia suberosa.. Planta Med 67: 572–572.
- Yang-Chang W, Chang-Yih D, Shang-Kwei W, Keh-Shaw C, Tsang-Hsiung Y (1990): Two new natural azafluorene alkaloids and a cytotoxic aporphine alkaloids from Polyalthis longifolia.. J Nat Prod 33: 1327–1327.
- Zafra-polo MC, González MC, Tormo JR, Estornell E, Cortes D (1996): Polyalthin: New prenylated benzopyran inhibitor of the mammalian mitochondrial respiratory chain. J Nat Prod 59: 913–916.