Abstract
The recent increase in the incidence of tuberculosis with the emergence of multidrug-resistant (MDR) cases has lead to the search for new drugs that are effective against MDR strains of Mycobacterium tuberculosis. and can augment the potential of existing drugs against tuberculosis. Pelargonium sidoides. DC (Geraniaceae) is highly valued by traditional healers for its curative properties and is well-known to treat coughs, diarrhea, and tuberculosis. The butanol root extract was found have bioactive inhibitory activity against M. tuberculosis. at a concentration of 2.5 × 103 µg/mL. Phytochemical analysis of the active fraction from the root of P. sidoides. led to the isolation and identification of six compounds: coumarins (umckalin, scopoletin, 6,8-dihydroxy-5.7-dimethoxy-2H.-benzopyran-2-one, and 6,8-dihydroxy-7-methoxy-2H.-benzopyran-2-one) and flavonoids (catechin and epigallocatechin, which is reported for the first time from P. sidoides.). The isolated compounds were evaluated for antitubercular activity with M. smegmatis. and M. tuberculosis.. Intracellular activity of these compounds was also investigated using THP-1 human macrophages infected with M. tuberculosis.. The isolated compounds did not show activity inhibitory against M. tuberculosis., intracellularly and extracellularly, at the highest concentration tested in the current study. Epigallocatechin and scopoletin showed good inhibitory activity against M. smegmatis., exhibiting a minimum inhibitory concentration (MIC) of 7.8 µg/mL. Catechin and umckalin exhibited MIC values of 31.25 and 62.5 µg/mL, respectively.
Introduction
Tuberculosis (TB) remains a major world health problem, in particular because the incidence of multidrug-resistant tuberculosis has increased in many countries (Cantrell et al., Citation1998). It is thought that as many as 2 million people have been exposed to the tuberculosis bacillus and are therefore at risk of developing the active disease. In addition, the high susceptibility of human immunodeficiency virus–infected persons to this illness and the proliferation of multidrug-resistant strains have created much scientific interest in developing new antimycobacterial agents (Gutierrez-Lugo et al., Citation2005). The breakdown in health services, the spread of HIV/AIDS, and the emergence of multidrug-resistant strains of Mycobacterium tuberculosis. (MDR) are contributing to the worsening impact of this disease (Bloom, Citation2002). Discovering new drugs represents a challenge, and it is hoped that new compounds can be found that will be effective against both the growing number of drug-resistant strains and against bacilli (Secrist et al., Citation2001).
An important consideration in the treatment of tuberculosis is the fact that the etiologic agent, M. tuberculosis., has the ability to persist intracellularly in the host macrophage for long periods of time (Quenelle et al., Citation2001). Optimum therapy, therefore, must depend upon the ability of the antituberculosis agent to eradicate the bacteria within the macrophage. This becomes even more important when one considers the ability of M. tuberculosis. to persist in a dormant state, thus giving rise to a large group of infected individuals who carry the organism in a subclinical state without having active disease (Collins, Citation1998). New drugs that act synergistically with the classic tuberculosis drugs within the host macrophages might have direct access to dormant organisms that presumably would be within macrophages or in the surrounding lymphatic area (Quenelle et al., Citation2001). First-line drugs used for the treatment of infections caused by M. tuberculosis. include isoniazid (INH), rifampicin (RMP), ethambutol (EMB), streptomycin (STR), and pyrazinamide (PZA) (Snider & La Montagne, Citation1994). Most in vitro. studies evaluate the efficacy of new drugs as single agents, but because, in clinical practice, a combination of various drugs is necessary to prevent the development of drug resistance during therapy, studies of drug combinations are therefore necessary (Bergmann & Woods, Citation1998).
In continuation of our laboratory's search for potential bioactive activity of Pelargonium. species (Geraniaceae), extracts and fractions of the root part of P. sidoides. DC were screened and subjected to isolation and tested against M. smegmatis. and M. tuberculosis.. M. tuberculosis. is a slow-growing and pathogenic organism. Therefore, samples were first screened against rapidly growing, nonpathogenic M. smegmatis. M. smegmatis. has similar drug sensitivity profiles to M. tuberculosis. and is an accepted target for selection of active molecules (Newton et al., Citation2002; Seidel & Taylor, Citation2004). Previous studies have investigated the anti-TB, antibacterial, and antifungal activity of two Pelargonium. species (Kolodziej, Citation2000; Latté & Kolodziej, Citation2000; Kolodziej et al., Citation2003; Mativandlela et al., Citation2006). We report on the isolation, identification, and antitubercular activity of isolated compounds obtained from the butanol fraction of P. sidoides. DC.
Materials and Methods
Plant material
Roots of P. sidoides. were collected in April and May from Qwaqwa, a region in the Free State province of South Africa. A voucher specimen (P 092559) was deposited and identified at the H.G.W.J. Schweickerdt Herbarium (PRU), University of Pretoria, South Africa.
Microorganisms and cell lines
A drug-susceptible strain of M. tuberculosis., H37Rv, obtained from American Type Culture Collection (ATCC, 27294), and M. smegmatis. MC2 155, were used to investigate the activity of the isolated compounds. THP-1 cell lines obtained from human peripheral blood monocytes were prepared in complete RPMI 1640 medium (Sigma-Aldrich Chemical Co., Johannesberg, South Africa).
Extraction and isolation
Fresh roots of P. sidoides. (1 kg) were extracted with 1 L of 100% ethanol for 2 h at room temperature; the residue was washed with fresh ethanol. The extracts were combined and concentrated to dryness at reduced pressure with a rotary evaporator. The residue (87 g) was dissolved in aqueous methanol and sequentially extracted with ethyl acetate and butanol. Antitubercular activity of ethyl acetate and butanol fractions was conducted against M. tuberculosis.. Because the butanol fraction showed antitubercular activity against M. tuberculosis., it was selected for isolation. The butanol fraction (15 g) was dissolved in 2 L of 100% methanol and subjected to a Sephadex LH-20 column using methanol as eluant. Fractions (1–66) of 500 mL were collected. Similar fractions were pooled together and dried, which resulted in three main fractions. Repeated chromatography on Sephadex LH-20 and preparative TLC of silica gel resulted in six pure compounds. The isolated compounds were identified mainly using 1H, 13C NMR in addition to a Distortionless Enhancement of Polarization Transfer (DEPT) experiment that compared with the reported data.
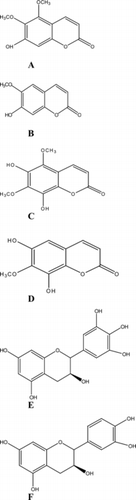
The compounds were identified as coumarins (umckalin, scopoletin, 6,8-dihydroxy-5,7-dimethoxy-2H.-benzopyran-2-one, and 6,8-dihydroxy-7-methoxy-2H.-benzopyran-2-one) and flavonoids (catechin and epigallocatechin, which is reported for the first time from P. sidoides.) ().
Figure 1 Coumarins and tannins isolated from butanol root fraction of P. sidoides.. (A) Umckalin; (B) scopoletin; (C) 6,8-dihydroxy-5,7-dimethoxy-2H.-benzopyran-2-one; (D) 6,8-dihydroxy-7-methoxy-2H.-benzopyran-2-one; (E) epigallocatechin; (F) catechin.
Microdilution assay using M. smegmatis
The bacterial strain of M. smegmatis. was cultured onto Middlebrook 7H11 agar base (Sigma-Aldrich Chemical Co., South Africa) and allowed to grow for 24 h at 37°C before use. The bacteria was carefully scraped and transferred into a sterilized glass tube containing a few glass beads (2 mm in diameter), and 10 mL of Middlebrook 7H9 broth base (Sigma-Aldrich Chemical Co., South Africa) was added to the culture. The suspension was homogenized by using a vortex mixer (Heidolph, REAX 2000, Labote, Johannesberg, Germany) and then left undisturbed for about 15 to 30 min to allow clumped bacteria to settle to the bottom of the tube. The supernatant was carefully transferred to an empty sterile tube and adjusted with Middlebrook 7H9 broth base to an optical density of 0.2 (log-phase) at 550 nm (Beckman DU-720 UV spectrophotometer, Beckman Coulter, Johannesburg, South Africa), yielding 1.26 × 108 colony-forming units per milliliter (CFU mL−1) (Newton et al., Citation2002).
The microdilution test was performed as used earlier by Newton et al. (Citation2002), in 96-well microtiter plates. The butanol extract and compounds (scopoletin, umckalin, epigalocatechin, and catechin) were dissolved in 10% dimethyl sulfoxide (DMSO) to obtain a concentration of 1 × 105 and 5 × 102 µg/mL, respectively. The other two isolated compounds (6,8-dihydroxy-5,7-dimethoxy-2H.-benzopyran-2-one and 6,8-dihydroxy-7-methoxy-2H.-benzopyran-2-one) could not be tested due to insufficient quantity. Ciprofloxacin (Sigma-Aldrich Chemical Co., South Africa) served as the positive drug control, at a concentration of 0.25 µg/mL against M. smegmatis. (Salie et al., Citation1996). Serial two-fold dilutions of each sample tested were made with Middlebrook 7H9 broth base to yield volumes of 100 µL/well with final concentration ranging from 2.5 to 0.0195 µg/mL for butanol extract and 125.0 to 0.97 µg/mL for compounds. M. smegmatis. (100 µL adjusted an optical density value, which would ensure the bacteria was at the start of the log phase when the test commenced) was also added to each well containing the samples and mixed thoroughly to give a final volume of 200 µL/well. The solvent control, DMSO at 2.5%, did not show inhibition on the growth of the bacteria. Tests were done in duplicate. The plates were sealed with parafilm and incubated at 37°C for 24 h. The minimum inhibitory concentration (MIC) of samples was detected following addition (40 µL) of 0.2 mg/mL p.-iodonitrotetrazolium chloride (INT; Sigma-Aldrich Chemical Co., South Africa) and incubated at 37°C for 30 min (Eloff, Citation1998). Viable bacteria reduced the yellow dye to a pink color. MIC was defined as the lowest sample concentration that prevented this change and exhibited complete inhibition of bacterial growth.
Antitubercular rapid radiometric assay using M. tuberculosis.
All the cultures of M. tuberculosis. were plated onto Löwenstein-Jensen medium and allowed to grow for 3–4 weeks at 37°C before use. Once there was satisfactory growth of bacteria on a Löwenstein-Jensen slant, bacteria were carefully scraped and transferred into a sterilized glass tube containing a few glass beads (2 mm in diameter). Tween 80/saline (5 mL of 0.04%) was added to the culture. The suspension was homogenized by using a vortex mixer (Heidolph, Germany) and then left undisturbed for about 15 to 30 min to allow clumped bacteria to settle to the bottom of the tube. The supernatant was carefully aspirated with a pipette, transferred to an empty sterile tube, and turbidity adjusted to approximate that of a McFarland no. 1 standard by adding 0.04% Tween 80/saline.
The radiometric respiratory techniques using the BACTEC 460 system (Becton Dickinson Diagnostic Instrument, Sparks, MD, USA) was used for susceptibility testing against M. tuberculosis. as described previously (Lall & Meyer, Citation2001; Lall, Citation2002; Lall et al., Citation2003; Mativandlela et al., Citation2006). Solutions of all the compounds were prepared in DMSO to obtain a concentration of 1 × 105 µg/mL. Control experiments showed that a final concentration of DMSO (1%) in the medium had no adverse effect on the growth of M. tuberculosis.. The primary drug isoniazid (INH) (Sigma-Aldrich Chemical Co., South Africa), at concentration of 0.02 µg/mL, served as drug-control in our bioassay. A homogenous culture of 0.1 mL of M. tuberculosis., yielding 1 × 104 to 1 × 105 CFU mL−1, was inoculated in the vials containing compounds as well as in the control vials (Heifets et al., Citation1985). Three compound-free vials were used as controls (medium + 1% DMSO); two vials (V1) were inoculated in the same way as the vials containing the compounds, and one (V2) was inoculated with a 1:100 dilution of the inoculum (1:100 control) to produce an initial concentration representing 1% of the bacterial population (1 × 102 to 1 × 103 CFU mL−1). The MIC against M. tuberculosis. was defined as the lowest concentration of the compound that inhibited >99% of the bacterial population.
Mycobacterium. growing in 7H12 medium containing 14C-labeled substrate (palmitic acid) uses the substrate and produced 14CO2. The amount of 14CO2 detected is expressed in terms of the growth index (GI) (Midlebrook et al., Citation1977). Inoculated bottles were incubated at 37°C, and each bottle was assayed every day to measure the GI at about the same hour(s) until cumulative results were interpretable.
Intracellular activity
Isolated compounds were further tested in THP-1 human macrophage cell line in order to evaluate the efficacy in real physiologic conditions. Final concentrations of test compounds were between 0.312 and 200 µg/mL and 0.120 µg/mL for INH. Culture of M. tuberculosis. was grown in 7H9 broth (supplemented with Middlebrook OADC enrichment; Sigma-Aldrich Chemical Co., South Africa), containing 0.05% (v/v) Tween 80 to avoid clumping at 37°C in a humidified 5% CO2 atmosphere and grown in 96-well plates to their exponential phase growth of an optical density of 0.15 corresponding to 108 CFU/mL, and 0.1 mL of the culture was added to 10 mL of RPMI 1640 medium (Rastogi et al., Citation1991). Culture of human macrophages (105 macrophages/well) were prepared from adherent peripheral blood monocytes from healthy donors and infected with tubercle bacilli at a ratio of 10 to 20 bacilli per cell. For infection, 1 mL of the bacteria medium was removed from each well and replaced with 1 mL of RPMI 1640 medium. The macrophages were allowed to phagocytize the bacteria for 4 h at 37°C, and the number of intracellular organisms was determined by lysing the macrophages with 0.25% (w/v) of sodium dodecyl sulfate (SDS), doing serial dilutions and plating the lysates on 7H11 agar medium for viable count determinations. After phagocytosis, fresh medium containing the desired antimicrobial agents was refed to macrophage-containing wells, and the culture was enumerated after lysing the macrophages at day 5. The results were compared with the growth of bacteria in the control culture (untreated macrophages). A compound was considered bactericidal if it effectively reduced the bacteria viable counts in the test sample compared with the initial inoculum added at the time of compound addition (Rastogi et al., Citation1991Citation1996).
Results and Discussion
The inhibitory activity of compounds against M. smegmatis. is depicted in . MIC value of butanol extract was found to be 0.156 µg/mL, umckalin was 62.5 µg/mL, catechin was 31.25 µg/mL, and scopoletin and epigalocatechin were 7.81 µg/mL. Seidel and Taylor (Citation2004) tested extracts and unsaturated compounds of both P. reniforme. Curtis and P. sidoides. against M. smegmatis. and M. aurum.. Only hexane extract and linoleic acid of P. reniforme. were found to inhibit M. aurum. at MIC values of 62.0 and 2.0 µg/mL, respectively. Other extracts possessed very weak activity or were found to be inactive. In another study, scopoletin, umckalin, 5,6,7-trimethoxycoumarin, (+)-catechin, gallic acid and its methyl ester, and 6,8-dihydroxycoumarin in P. sidoides. were evaluated against eight microorganisms, including three Gram-positive (Stophylococcus aureus., Streptococcus pneumoniae., and β.-hemolytic streptococcus) and five Gram-negative (Escherichia coli., Klebsiella pneumonia., Proteus mirabilis., Pseudomonas aeruginosa., Haemuphilus influenzae.), at MIC values of 5 × 103 to 7.5 × 103 µg/mL. All compounds exhibited more or less pronounced antibacterial activities against Gram-positive and Gram-negative pathogens exhibiting MIC values of 2 × 102 to 2 × 103 µg/mL. Umckalin and 6,8-dihydroxy-5,7-dimethoxycoumarin showed antibacterial activity, and MIC ranged from 3 × 102 to 5 × 102 µg/mL (Kolodziej et al., Citation2003).
Table 1.. Activity of extract and compounds of P. sidoides. against M. smegmatis. using the microdilution method.
All six isolated compounds did not exhibit antimycobacterial activity against M. tuberculosis. at the highest concentration tested (200 µg/mL). All the compounds were also found not active against M. tuberculosis.–infected THP-1 cell lines, as none of the treatments led to a ΔGI less than the ΔGI of the 1:100 control. None of the compounds tested proved to be bactericidal as none led to a reduction in viable bacterial counts of 90% or more compared with the phagocytosis control (Rastogi et al., Citation1996). Similar results were obtained by Kolodziej et al. (Citation2003), where none of the isolated phenolic coumarins showed in vitro. antimycobacterial activity. These observations by us and Kolodziej et al. (Citation2003) on antimycobacterial activity suggest that the coumarins are not active against M. tuberculosis.. Anand et al. (Citation2006) investigated epigallocatechin-3-gallate from green tea and there was 18% inhibition of M. tuberculosis. survival within macrophages. However, no compounds from Pelargonium. extracts with antimycobacterial activity have so far been reported.
Therefore, it is speculated that the anecdotal evidence of tuberculosis patients could be due to direct or indirect effects. Other compounds present in P. sidoides. that have not been isolated in this study or in previous studies done by scientists or researchers might be responsible for the curative effect of Pelargonium. extracts in TB patients.
Acknowledgments
The authors are grateful to the technical assistants of the Medical Research Council (Pretoria, South Africa) and to the National Research Foundation for financial support.
References
- Anand PJ, Deepak K, Sharma M (2006): Green tea polyphenol inhibits Mycobacterium tuberculosis. survival within human macrophages. Int J Biochem Cell B 38: 600–609.
- Bergmann JS, Woods GL (1998): In vitro. activity of antimicrobial combinations against clinical isolates of susceptible and resistant Mycobacterium tuberculosis.. Int J Tuberc Lung D 2: 621–626.
- Bloom BR (2002): Tuberculosis-the global view. Medicine 346: 1434–1435.
- Cantrell CL, Fischer NH, Urbatsch L, McGurie MS, Franzblau SG (1998): Antimycobacterial crude plant extracts from South, Central and North America. Phytomedicine 5: 137–145.
- Collins FM (1998): Tuberculosis research in a cold climate. Tubercle Lung Dis 78: 99–107.
- Eloff JN (1998): A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extract for bacteria. Planta Med 64: 711–713.
- Gutierrez-Lugo MT, Wang Y, Franzblau SG, Suarez E, Timmermann BN (2005): Antitubercular sterols from Thalia multiflora. Horkel ex Koernicke. Phytother Res 19: 876–880.
- Heifets LB, Iseman MD, Cook JL, Lindholm-Levy PJ, Drupa I (1985): Determination of in vitro. susceptibility of Mycobacterium tuberculosis. to cephalosporins by radiometric and conventional methods. Antimicrob Agents Chemother 27: 11–15.
- Kolodziej H (2000): Traditionally used Pelargonium. species: Chemistry and biological activity of umckaloabo extracts and their constituents. Curr Topics Phytochem 3: 77–93.
- Kolodziej H, Kayser O, Radtke O, Kiderlen A, Koch E (2003): Pharmacologicalprofile of extracts of Pelargonium sidoides. and their constituents. Phytomedicine 10 (Suppl. IV): 18–24.
- Lall N (2002): Isolation and identification of naphthoquinones from Euclea natalensis. with activity against Mycobacterium tuberculosis., other pathogenic bacteria and Herpes simplex virus. PhD thesis, University of Pretoria.
- Lall N, Meyer JJM (2001): Inhibition of drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis. by diospyrin isolated from Euclea natalensis.. J Ethnopharmacol 78: 213–219.
- Lall N, Das Sarma M, Hazra B, Meyer JJM (2003): Antimycobacterial activity of diospyrin derivatives and a structural analogue of diospyrin against Mycobacterium tuberculosis in vitro.. J Antimicrob Chemother 51: 435–438.
- Latté KP, Kolodziej H (2000): Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Zeitschrift für Naturforschung (C) 55: 467–472.
- Mativandlela SPN, Lall N, Meyer JJM (2006): Antibacterial, antifungal and antitubercular activity of the roots of Pelargonium reniforme. (CURT) and Pelargonium sidoides. (DC) (Geraniaceae) root extracts. S Afr J Bot 72: 232–237.
- Middlebrook G, Reggiards Z, Tigertt WD (1977): Automable radiometric detection of growth of Mycobacterium tuberculosis. in selective media. Am Rev Respir Dis 115: 1067–1069.
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW (2002): The evaluation of forty-three plant species for in vitro. antimycobacterial activities; isolation of active constituents from Psoralea corylifolia. and Sanguinaria canadensis.. J Ethnopharmacol 79: 57–67.
- Quenelle DC, Winchester GA, Staas JK (2001): Treatment of tuberculosis using a combination of sustained-release rifampin-loaded microspheres and oral dosing with isoniazid. Antimicrob Agents Chemother 45: 1637–1644.
- Rastogi N, Labrousse V, Goh KS, Carvalho De Sousa JP (1991): Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with otherdrugs against Myocabacterium avium. complex growing extracellularly and intracellularly in marine and human macrophages. Antimicrob Agents Chemother 35: 2473–2480.
- Rastogi N, Labrousse V, Seng Goh KS (1996): In vitro. activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis. and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol 3: 49–56.
- Salie F, Eagles PFK, Leng HMJ (1996): Preliminary antimicrobial screening of four South African Asteraceae species. J Ethnopharmacol 52: 27–33.
- Secrist J, Anathan S, Kwong C (2001): Search drugs for treatment of tuberculosis. Antimicrob Agents Chemother 45: 1943–1946.
- Seidel V, Taylor PW (2004): In vitro. activity of extracts and constituents of Pelargonium. against rapidly growing mycobacteria. J Antimicrob Agents 23: 613–619.
- Snider DE, La Montagne JR (1994): The neglected global tuberculosis problem: A report of the 1992 World Congress on tuberculosis. J Infect Dis 169: 1189–1196.
