Abstract
Acorus calamus. Linn. (Araceae), commonly known as “sweet flag” or “calamus”, is a semiaquatic, perennial, aromatic herb with creeping rhizomes. The plant is found in the northern temperate and subtropical regions of Asia, North America, and Europe. The plant exhibits polyploidy. Many ethnomedicinal and ethnobotanical uses have been ascribed to the rhizomes of the plant. A. calamus. Linn. (AC) has been used as traditional Chinese and Indian prescriptions for its beneficial effects on memory disorder, learning performance, lipid peroxide content, and anti-aging and anticholinergic activity. Moreover, pharmacological studies have revealed that Acorus. rhizome and its constituents, particularly α.- and β.-asarone, possess a wide range of pharmacological activities such as sedative, CNS depressant, behavior modifying, anticonvulsant, acetylcholinesterase inhibitory, memory enhancing, anti-inflammatory, antioxidant, antispasmodic, cardiovascular, hypolipidemic, immunosuppressive, cytoprotective, antidiarrheal, antimicrobial, anthelmintic, insecticidal, adulticidal, diuretic, antioxidant, genotoxic, and mutagenic activities. This review is an effort to explore the different phytoconstituents and pharmacological activities of Acorus calamus..
Introduction
Acorus calamus. (AC) Linn. (Araceae), commonly known as “sweet flag” or “calamus”, is a species of semiaquatic, perennial, aromatic herb with creeping rhizomes. The plant is found in the northern temperate and subtropical regions of Asia, North America, and Europe. The plant prefers swampy or marshy habitats. The plant exhibits polyploidy and three karyotypes. The diploid karyotype (2n = 24) grows in North America and in parts of Asia (Siberia); the triploid karyotype (3n = 36) is present in Central Europe and Kashmir, India; the tetraploid karyotype (4n = 48) is found in India, East Asia, and Japan. In India, the plant is found growing wild as well as cultivated up to an altitude of 2200 m in the Himalayas. It is plentiful in the marshy tracts of Kashmir, Himachal Pradesh, Manipur, and Naga hills, and is regularly cultivated in Karnataka (Cavazza, Citation1976).
Acorus calamus. Linn. (AC) possesses grass-like or sword-shaped, long slender leaves that fan out from a pinkish base and grow up to 1.5 m in length. The midvein is usually off-center. Cut or bruised leaves produce a sweet, tangerine-like scent. The flower stem, or scape, arises from the base of the outer leaves. The slightly curved spadix is crowded with small yellowish-green to brown flowers. The plant bears green, angular, 1 to 3 seeded berries. The seeds are oblong in shape (Anonymous, Citation2001). The rhizomes of the plant are the most important part. The rhizomes are woody, branched, light-brown or occasionally orange-brown in color, cylindrical to flat with distinct nodes and internodes. The rhizomes possess strong, characteristic, and aromatic odor and are bitter in taste (Anonymous, Citation2001). The transversely cut surface of the rhizome is cream in color with pinkish tinge and differentiated into narrow cortical and large stelar regions. Microscopically, epidermis has radially elongated cells with heavily thickened walls. The cortical region consists of thin-walled parenchymatous cells in chains, having large intercellular spaces, sheathed collateral vascular bundles, and patches of fibers. The stelar region has single barrel-shaped endodermal cells with abundant starch grains. Endodermal-cells are barrel-shaped and possess abundant starch grains. Large oil cells with yellowish content, cells containing dark-brown oleoresin content, and starch grains are scattered in the ground tissue of both cortex and stele. Solitary polygonal crystals of calcium oxalate are present in each cell of the storied row of cells running parallel to the fibers.
Various therapeutic potentials have been attributed to AC rhizomes in the traditional system of medicine as well as in folklore practices. Various bioactive phytoconstituents and pharmacological activities of the plant have been reported over the years. Hence, this review is directed toward exploring the various pharmacological activities of AC rhizomes as well as the phytoconstituents.
Medicinal Uses
Uses ascribed in traditional medicine
In the Ayurvedic system of medicine, the rhizomes of AC are considered to possess aromatic, stimulant, bitter tonic, emetic, expectorant, emmenagogue, aphrodisiac, laxative, diuretic, antispasmodic, carminative, and anthelmintic properties. They are used for the treatment of a host of diseases such as mental ailments like epilepsy, schizophrenia, and memory disorders, chronic diarrhea and dysentery, bronchial catarrh, intermittent fevers, tympanitis, colic, otitis media, cough, asthma, and glandular and abdominal tumors (Kirtikar & Basu, Citation1987; Anonymous, Citation2001). They are also used traditionally for flatulent colic and chronic dyspepsia. They are also employed for kidney and liver troubles, rheumatism, and eczema. The skin of the rhizomes is said to be hemostatic. The rhizomes are used in the form of powder, balms, enemas, and pills and also in ghee preparations (Kirtikar & Basu, Citation1987; Anonymous, Citation2001).
Uses described in folklore medicine
The rhizome is used as an emetic (Srivastava et al., Citation1986) and to kill lice (Megoneitso & Rao, Citation1983). The leaves are used on wounds to kill worms (Kapur, Citation1993). The stem is used in cough, cold (Megoneitso & Rao, Citation1983), and toothache. The rhizome is useful in diseases of nervous system, throat, and diarrheal diseases and as an antitumor agent (Viswanathan, Citation1995; Jha & Verma, Citation1996). It is also used for protection against smallpox (Jain, Citation1989), ringworm (Bajpai et al., Citation1995), in respiratory and gastrointestinal tract diseases and snakebite (Bist & Badoni, Citation1990; Singh, Citation1995; Mohanty et al., Citation1996), in gout and rheumatism (Jain & Puri, Citation1994), and in dysmenorrhea (Borthakur & Goswami, Citation1995). The root is used in intermittent fever, as an antipyretic and antitussive (Dobriyal et al., Citation1997). It is also found useful in stomachache (Mao, Citation1993), and root bark is used as an antidote to snakebite (Singh & Prakash, Citation1994). The tribals in the Garhwal region of the Himalayas take a decoction of the rhizome as a nonalcoholic beverage. The fresh rhizomes are chewed to prevent intoxication from alcohol. The decoction is also given to children for gastroenteritis. The rhizome pieces, are tied around the belly for jaundice (Bist & Badoni, Citation1990). Natives of Tirumala hills also use the rhizomes for the treatment of dental disorders (Balaji Rao et al., Citation1996).
Ethnobotanical Uses Other Than Medicinal Uses
Since antiquity, AC rhizome has been used for medicinal baths, in incense, and for tea. The powdered rhizome is used as an insecticide for the destruction of fleas, bedbugs, moths, lice, and so forth. The rhizomes are used in incense sticks (Vashist & Handa, Citation1964). It is effective in killing insect pests in stored rice and is considered to be better than chemicals for this purpose as it shows no residual effect. The root is used as an insecticide and for protection from insect attacks (Jain & Suri, Citation1980; Singh et al., Citation1996).
Chemical constituents
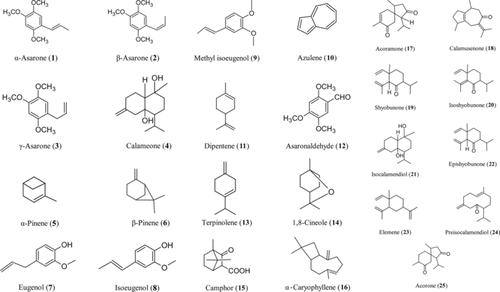
A wide variety of chemical constituents have been reported from the rhizomes of AC. The oil of AC rhizomes has been analyzed by various workers for their chemical constituents (Oprean et al., Citation2001; Raina et al., Citation2003). The oil was found to contain varying concentrations of α.-asarone (1), β.-asarone (2), γ.-asarone (3), calamene, calamenenol, calameone (4), α.-pinene (5), β.-pinene (6), camphene, p.-cymene, eugenyl acetate, eugenol (7), isoeugenol (8), methyl isoeugenol (9), calamol, azulene (10), eugenol methyl ether, dipentene (11), methyleugenol, asaronaldehyde (12), terpinolene (13), 1,8-cineole (14), camphor (15), α.-caryophyllene (16), and hydrocarbons (Fig.1) (Nigam et al., Citation1990; Srivastava et al., Citation1997; Mukherjee, Citation2002). The oil also contains fatty acids such as palmitic acid and its ester, heptylic acid, an ester of butyric acid (Chaudhury et al., Citation1957).
Sharma and Dandiya (Citation1969) first reported the synthesis of asarone from 1,2,4-trimethoxybenzene. Fractionation from the volatile oil by gas chromatography resulted in the isolation of α.-asarone and β.-asarone, which are the trans.- and cis.-isomers, respectively, of 2,4,5-trimethoxy-l-propenylbenzene (Baxter et al., Citation1960). Other constituents identified in the rhizome were cyclobutanolignan acoradin, 2,4,5-trimethoxybenzaldehyde, 2,5-dimethoxybenzoquinone, galangin (5,7-dihydroxytlavanol), along with sitosterol and acoramone (17) (Patra & Mitra, Citation1981).
Two phenyl indanes, viz., 2,3-(2,4,5-trimethoxyphenyl)-2-propenal and 2,3-dihydro-4,5,7-trimethoxy-1-ethyl-2-methyl-3 (2,4,5-trimethoxyphenyl) indane (Saxena, Citation1986) and two new triterpenoid saponins, 1β.,2α.,3β.,19α.-tetrahydroxyurs-12-en-28-oic acid-28-O.-{(β.-D-glucopyranosyl (1-2)}-β.-D galactopyranoside and 3-β., 22-α.-24,29-tetrahydroxyolean-12-en-3-O.-(β.-D arabinosyl (1,3)}-β.-D-arabinopyranoside, were characterized in the ethanol extract of the rhizome (Rai et al., Citation1998). The petroleum ether and benzene fractions of the rhizome were reported to yield thymol, cumin, and phellandrene, while the chloroform fraction yielded a triterpene alcohol and a triterpene acid (Negi & Joshi, Citation1981). The root was found to contain 13 amino acids of which arginine, lysine, phenylalanine, threonine, and tryptophan were essential amino acids. The other amino acids identified were α.-alanine, asparagine, aspartic acid, glutamic acid, norvaline, proline, and tyrosine (Vashi & Patel, Citation1987). The ethanol (50%) extract of the rhizome was found to contain glycosides, sterols, and terpenoids (Tewari et al., Citation1984). Two sesquiterpenic ketones of the guaiane-type calamusenone (18) and its isomer were isolated from sweet flag oil. Sesquiterpenes, namely shyobunone (19), isoshyobunone (20), isocalamendiol (21), dehydroxyisocalamendiol, and epishyobunone (22), were isolated from AC, in addition to calameone. The thermal isomerization of shyobunone (23), an elemene type sesquiterpene, resulted in the formation of preisocalamendiol (24), a germacrone-type compound, and acorone (25) (Yamamura et al., Citation1971; Nawamaki & Kuroyanagi, Citation1996). The structures of the major chemical constituents of Acorus. rhizomes are shown in .
Pharmacological Activities
AC has been screened for various pharmacological activities. It has significant CNS actions such as anticonvulsant, sedative, hypnotic, tranquilizing, and memory enhancing, which justifies its use in some CNS diseases in the Ayurvedic system of medicine. It also has effective acetylcholinesterase inhibitory, antispasmodic, antimicrobial, anti-inflammatory, anthelmintic, and insecticidal effects. The various pharmacological activities of AC are shown in .
Table 1.. Pharmacological activities of the rhizome of Acorus calamus. L.
Sedative and hypnotic effect
The volatile oils from AC showed potentiation of the sedative activity of pentobarbitone in mice. The active principle responsible for the activity resided in the hydrocarbon fraction of the oil or in an oxygenated component out of various fractions of the oil (Dandiya et al., Citation1959a). The steam volatile fractions prolonged the sleeping time in mice with pentobarbital, hexobarbital, and ethanol. The sedative potentiating activity was highest in the volatile fraction of the petroleum ether extract (Dandiya & Cullumbine, Citation1959). Pretreatment of mice with lysergic acid diethylamide (LSD) partly prevented the hypnotic potentiating action of the volatile oil (Dandiya et al., Citation1959b). Study on the mechanism of the hypnotic potentiation of barbiturate-induced hypnosis in mice by acorus. oil showed that the potentiating action was antagonized by lysergic acid diethylamide as well as dibenzyline hydrochloride. The results proved that the hypnotic potentiating action might be mediated through serotonin and catecholamines (Malhotra et al., Citation1962). CNS of the essential oil showed sedative-tranquilizing action in rats, mice, cats, dogs, and forced motor activities in mice. The oil inhibited monoamine oxidase at a higher dose level (Dhalla & Bhattacharya, Citation1968). Essential oil from the rhizome of AC antagonized amphetamine-induced agitational symptoms and also inhibited the conditioned avoidance response in rats (Bhattacharya, Citation1968). β.-Asarone exerts sedative and hypothermic effects in rats. In one study, the alcohol extract of AC showed potent sedative and analgesic properties. The essential oil, crude alcohol, and aqueous extracts showed depressant action in dogs (Bose et al., Citation1960).
CNS depressant activities
The effect of the ethanol extract of AC was studied on spontaneous electrical activity and monoamine levels of the brain. In AC-treated rats, electrogram recording revealed there was an increase in α. activity together with an increase in the norepinephrine level in the cerebral cortex, but a decrease in the midbrain and cerebellum. The serotonin level was increased in the cerebral cortex but decreased in the midbrain. Similarly, the dopamine level was increased in the caudate nucleus and midbrain but decreased in the cerebellum. Thus, AC showed its depressive action by changing electrical activity and by differentially altering brain monoamine levels in different brain regions (Hazra & Guha, Citation2003). Tripathi and Singh (Citation1995) performed a clinical study in 50 cases of depression. AC given for 6 weeks showed reduction in the degree of severity of depression and better rehabilitation and also a significant improvement in assessment based on the rating of symptoms on the Hamilton depression rating scale.
α.-Asarone and β.-asarone showed many pharmacodynamic actions similar to some well-established tranquilizers. α.-Asarone and β.-asarone significantly enhanced the anesthetic activity of pentobarbitone, hexobarbitol, and ethanol in mice. β.-Asarone appeared to be more active (Sharma et al., Citation1961). The mechanism of tranquilizing action of α.-asarone was also studied. α.-Asarone was not found to cause any change in the noradrenaline content of whole brain of rat, and pretreatment of α.-asarone failed to block the effect of reserpine on the spontaneous motor activity and ptosis of mice, as well as the conditioned avoidance response of trained rats. It was found that the sedative effect of α.-asarone was dependent on the depression of the ergotropic division of the hypothalamus (Menon & Dandiya, Citation1967). β.-Asarone administered in association with a cannabinomimetic drug was shown to potentiate some of the typical behavioral activities induced in animals by cannabinoids (Zanoli et al., Citation1998). α.-Asarone reduced spontaneous motor activity and caused reduction in anxiety without dulling the perception in rats. It produced a prolonged calming effect in monkeys (Dandiya & Menon, Citation1964). α.-Asarone also partially antagonized tremorine-induced tremors in mice but was found to be inferior to atropine in this respect. The tranquilizing effect of α.-asarone was responsible for its antagonism to mescaline and amphetamine, while the anticholinergic effect accounted for the partial protection offered to tremorine-treated mice (Dandiya & Menon, Citation1965).
Anticonvulsant activity
The steam volatile fractions of rhizome of AC exacerbated tonic seizures provoked by metrazol in rats and also potentiated the action of reserpine in reducing amphetamine toxicity in aggregated mice (Dandiya & Cullumbine, Citation1959). Acorus. oil investigated for its antianaleptic activity was used as a saline suspension, given 1 h prior to production of convulsions in adult albino mice. It successfully prevented seizures in maximal electroshock seizures test (Khare & Sharma, Citation1982). β.-Asarone caused generalized convulsion and potentiated metrazol seizures in rats, while α.-asarone showed a tendency to protect against metrazol convulsions and modified electroshocks (Sharma et al., Citation1961). In a study using electroconvulsions, α.-asarone increased the percentage mortality of animals treated with chlorpromazine but not of those treated with reserpine (Dandiya & Sharma, Citation1962; Dandiya & Menon, Citation1963). The aqueous and alcohol extracts were found to reduce the severity of maximum electric shock–induced seizure in rats. Further, the extracts significantly increased the pentylenetetrazole-induced seizure latency (Manis et al., Citation1991). The essential oil showed a protective effect against electroshock seizures in rats (Madan et al., Citation1960).
Behavorial changes
The steam volatile fractions of the rhizome of AC produced a fall in the blood pressure of anesthetized cats, caused hypothermia in mice, and potentiated the action of acetylcholine, histamine, and barium chloride on the isolated gut of guinea pigs (Dandiya & Cullumbine, Citation1959). The in vitro. studies of the oil on the respiration of rat brain revealed that it inhibited the oxygen uptake of brain tissues (Dhalla et al., Citation1961). The oil affects the 5-HT and noradrenaline contents of brain similar to reserpine (Malhotra et al., Citation1961). Estimation of brain 5-HT content of rat revealed that neither the oil nor its active principles liberated 5-HT. These compounds also did not cause additional decrease of the brain 5-HT content in reserpine-treated animals (Dandiya & Sharma, Citation1962; Dandiya & Menon, Citation1963;). The oil also caused hypothermia in mice and, even in low doses, counteracted LSD-induced hyperpyrexia. α.-Asarone failed to cause release of 5-HT from the brain and also prevented the depletion of adrenal ascorbic acid in rats subjected to cold stress (Dandiya & Menon, Citation1964). The effect of α.-asarone was studied on experimentally induced conflict neurosis in rats, and it was found to increase the number of shocks accepted by the animals (Chak & Sharma, Citation1965).
In one study, α.-asarone antagonized the hyperactivity and hallucinogenic effect of mescaline in rats and offered protection to aggregated mice treated with dextro. amphetamine. Intraperitoneal administration of a flavone isolated from the chloroform extract of the rhizome produced profound behavioral changes in the rhesus monkey. This flavone also produced a calming effect on conscious animals, viz.., mice, rats, and rabbits. The flavone showed an activity similar to Cannabis indica. (Dasgupta et al.,Citation1977). A study was conducted with crude powder administered for 60 days to investigate the effects of the plant on escape/avoidance conditioning and general motor activity of rats. It enhanced learning performance, especially in the females, whereas the effect of the plant on general activity was not significant (Singh, Citation1989). The ethanol extract of the rhizome was screened for CNS activity in mice and rats. The extract exhibited a large number of actions similar to α.-asarone but differed from the latter in several respects including the response to electroshock, apomorphine and isolation-induced aggressive behavior, amphetamine-toxicity in aggregated mice, and behavioral despair syndrome in forced swimming (Vohora et al.,Citation1990). In one study, the essential oil of AC was tested for its effect on motor activity, the position of the eyelids, and the general state. General depression without ataxia was observed (Shipochliev et al., Citation1968).
In one study, exposure of rats to acrylamide caused hind limb paralysis in 58% of the animals on day 10 and decreased behavioral parameters, namely distance traveled, ambulatory time, stereotypic time, and basal stereotypic movements compared with the control group. Treatment with the ethanol:water (1:1) extract of the rhizomes of AC increased the GSH content and GST activity in the corpus striatum where as insignificant changes were observed in other parameters. The rats also showed a partial recovery in other behavioral parameters. The levels of GSH content and GST activity increased in the corpus striatum, where as the dopamine receptors decreased compared with the AC-treated rats. The results proved that the neurobehavioral changes produced by acrylamide was prevented with the treatment by ACrhizomes (Shukla et al., Citation2002). The neuroprotective potential of ethanol:water (1:1) extract of rhizomes of AC was reported in middle cerebral artery occlusion (MCAO)-induced ischemia in rats. Ischemic rats treated with AC exhibited a significant improvement in neurobehavioral performance, viz.., rota-rod performance and grid walking. Extract treatment significantly decreased malonaldialdehyde levels in cortex, increased reduced glutathione levels and SOD activity in both cortex and corpus striatum, and neurologic function score was also improved in the AC-treated rats (Shukla et al., Citation2006).
Acetylcholinesterase-inhibitory and memory-enhancing effect
In Ayurveda, herbal medicines with rasayana. effects are believed to be restorative, to attain longevity, intelligence, and freedom from age-related disorders. AC is regarded in Ayurvedic medicine as promoting rasayana effects and has been used to treat memory loss (Kirtikar & Basu, Citation1954; Mukherjee & Wahile, Citation2006a). AC is used in Ayurvedic medicine on a regular basis for the treatment of memory loss and other mental disorders (Kirtikar & Basu, Citation1954; Howes & Houghton., Citation2003). AC extract has also been used as traditional Chinese prescription, and its beneficial effects on memory disorder, on learning performance, lipid peroxide content, and anti-aging effects in senescence have been reported (Nishiyama et al., Citation1994; Zhang et al., Citation1994). The in vitro. acetylcholinesterase (AChE) inhibitory effect of hydro- alcohol extract and essential oil of AC rhizomes was reported based on Ellman's method in 96-well microplates using bovine erythrocytes. The essential oil showed stronger inhibition than the hydro-alcohol extract (Houghton et al., Citation2006a). Methanol extracts of AC showed significant acetylcholinesterase enzyme inhibition at a concentration 200 µg/mL (Oh et al., Citation2004). Houghton et al. (Citation2006b) reported the in vitro. acetylcholinesterase inhibitory effect of β.-asarone and α.-asarone. β.-Asarone isat least an order of magnitude more active than its trans.-isomer α.-asarone. The AChE-inhibitory activity of the oil can be ascribed to β.-asarone. Because cognitive performance and memory are related to acetylcholine levels, the AChE-inhibitory effect of the plant may account for its traditional use.
Anti-inflammatory activity
Varde et al. (Citation1988) studied the anti-inflammatory activity of AC in rats using acute and chronic experimental models. The oral administration of the extract showed inhibition of the carrageenin-induced paw edema, inhibition of cotton pellet granuloma formation, and inhibition of croton oil granuloma pouch inflammatory response. The rhizomes extract showed significant anti-inflammatory effect in acute, chronic, and immunologic models of inflammation (Vohora et al., Citation1989).
Antioxidant activity
The ethyl acetate extract of AC was found to be a potent antioxidant by inhibition of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical (Acuna et al., Citation2002). In vitro. antioxidant activity by DPPH scavenging at three different concentrations (0.2, 0.1, and 0.01 g/mL) showed a maximum activity of 86.43% at 0.2 g/mL (Govindarajan et al., Citation2003). The ethyl acetate and methanol extracts of AC protected most of the changes of oxidative stress status in the rat brain induced by noise-stress. The results of this study proved that during exposure to noisy environment, ROS generation led to increase in corticosterone, LPO, and SOD, but a decrease in CAT, GPx, GSH, protein thiols, and vitamins C and E levels. Both the ethyl acetate and methanol extracts of Acorus calamus. protected against most of the changes in the rat brain induced by noise-stress (Manikandan et al., Citation2005).
Manikandan and Devi (Citation2005) demonstrated antioxidant properties of α.-asarone against noise-stress–induced changes in the rat brain. In their study, α.-asarone, one of the active principle components, was administered intraperitoneally one-half hour before the animals were exposed to noise-stress for 30 days; and they investigated whether a 30-day exposure to noise can produce an oxidative stress. The antioxidant was verified by measuring the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), levels of reduced glutathione (GSH), vitamin C, vitamin E, protein thiols and lipid peroxidation (LPO) in different regions of the rat brain. α.-Asarone had an effective protective role by normalizing the increased SOD and LPO, and decreasing CAT, GPx, GSH, vitamins C and E, and protein thiols due to noise exposure.
Actions on cardiovascular system
In one study, Chopra et al. (Citation1954) reported that the essential oil of AC caused a moderate depression of blood pressure. The essential oil showed quinidine-like activity in combating auricular fibrillation, auricular flutter, and ventricular arrhythmias after two-stage coronary ligation in dogs. It further resembled quinidine qualitatively in causing a prolongation of conduction time and refractory period in isolated rabbit auricles (Madan et al., Citation1960). The essential oil was reported to have negative inotropic and antiarrhythmic properties. α.-Asarone and β.-asarone showed cardiac depressant activity on frog and rabbit heart perfusion experiments and also possessed a moderate amount of hypotensive activity in anesthetized dogs (Arora, Citation1965). The alcohol extract (50%) of AC exhibited a dose-dependent hypotensive action on dog blood pressure (Moholkar et al., Citation1975). Mamgain and Singh (Citation1994) performed a clinical trial on 45 patients of ischemic heart disease. The efficacy of the AC was tested and showed a significant improvement in the AC-treated groups. AC was found to be effective in the improvement of chest pain, dyspnea on effort, reduction of body weight index, improving ECG, decreasing serum cholesterol, decreasing SLDL (serum low-density lipoproteins), and increasing SHDL (serum high-density lipoproteins).
Hypolipidemic activity
Administration of the ethanol (50%) extract of the rhizomes of AC (100 and 200 mg/kg) as well as saponins (10 mg/kg) isolated from the extract demonstrated significant hypolipidemic activity. On the contrary, the aqueous extract showed hypolipidemic activity only at a dose of 200 mg/kg (Parab & Mengi, Citation2002). α.-Asarone obtained from Acorus. rhizomes was also reported to possess hypolipidemic activity in mice (Garduno et al., Citation1997; Mukherjee, Citation2003).
Actions on respiratory system
The alcohol extract of rhizome possesses a bronchodilator effect (Bose et al., Citation1960). In a clinical trial of patients with moderate to severe bronchial asthma, the fresh rhizome of AC was administered by a chewing method for 2–4 weeks. The AC was found to have antiasthmatic potential without any side effects (Rajasekharan & Srivastava, Citation1977). In another study, small pieces of the rhizome were administered to asthmatic patients by a chewing method, and significant effect in relieving of bronchospasm was observed without any side effects (Chandra, Citation1980).
Anticancer activity
Two lectins purified from the rhizomes of two sweet flag species, namely AC, by affinity chromatography, showed potent antimitogenic activity toward mouse splenocytes and human lymphocytes. Both lectins also significantly inhibited the growth of J774, a murine macrophage cancer cell line and, to a lesser extent, WEHI-279, a B-cell lymphoma (Bains et al., Citation2005). The in vitro. anticellular property of the ethanol extract of AC rhizome was evaluated, and it was found that the extract inhibited proliferation induced by the mitogen phytohemagglutinin. In addition, AC extract inhibited growth of several cell lines of mouse and human origin. It also inhibited production of nitric oxide, interleukin-2, and tumor necrosis factor-α. (Mehrotra et al., Citation2003).
Immunosuppressive activity
The in vitro. immunomodulatory property of the ethanol extract of AC rhizome was evaluated. The extract was found to inhibit antigen (purified protein derivative) stimulated human peripheral blood mononuclear cells. Intracytoplasmic interferon-γ. (IFN-γ.) and expression of cell surface markers, CD16 and HLA-DR, on human peripheral blood mononuclear cells were not affected on treatment with AC extract, but CD25 expression was downregulated (Mehrotra et al., Citation2003).
Antiulcer and cytoprotective properties
The ethanol extract of AC was studied in rats for its ability to inhibit gastric secretion and to protect gastroduodenal mucosa against the injuries caused by pyloric ligation, indomethacin, reserpine, and cysteamine administration and cytodestructive agents including 80% ethanol, 0.6 M HCl, 0.2 M NaOH, and 25% NaCl. An oral dose of 500 mg/kg of the extract showed significant antisecretory and anti-ulcerogenic activity in rats subjected to pyloric ligation, indomethacin, reserpine, and cysteamine administration. The extract had a highly significant protective effect against cytodestructive agents. These findings support the use of calamus for the treatment of gastropathy in traditional medicine (Rafatullah et al., Citation1994).
Antidiarrheal activity
A study was undertaken to evaluate the effect of aqueous and methanol extracts of AC rhizome for its antidiarrheal potential against castor oil–induced diarrhea in mice. The methanol plant extract was more effective than the aqueous plant extract against castor oil–induced diarrhea. The methanol extract significantly reduced induction time of diarrhea and total weight of the feces (Shoba & Thomas, Citation2001).
Antispasmodic activity
The oil of AC rhizhome produced antispasmodic action on the involuntary muscle tissue by inhibiting the excessive peristaltic movements of the intestines in rabbits and dogs (Chopra et al., Citation1954). The alcohol extract showed relaxation of the smooth muscles in an isolated preparation of rat intestines and caused negative inotropic action on frog heart (Agarwal et al., Citation1956). The essential oil was found to be more effective than the alcohol and aqueous extracts, as observed in lung perfusion and isolated tracheal chain experiments (Bose et al., Citation1960). α.-Asarone and the essential oil from the rhizome showed a relaxant effect as well as antispasmodic effect against various spasmogens. The antispasmodic activity of α.-asarone indicated that the action was nonspecific and probably due to direct musculotropic action. α.-Asarone was more active than that of the essential oil (Das et al., Citation1962). In a another study, the ethanol (50%) extract of the rhizome showed antispasmodic activity on isolated guinea pig illeum and gross behavioral effect, hypothermia, and analgesic activity in mice (Bhakuni et al., Citation1988).
Another study by Gilani et al. (Citation2006) determined its possible pharmacological basis as an antispasmodic and antidiarrheal. In the isolated rabbit jejunum preparation, the crude extract of AC caused inhibition of spontaneous and high K+(80 mM)-induced contractions, with respective EC50 values of 0.42 ± 0.06 and 0.13 ± 0.04 mg/mL (mean ± SEM; n = 6 to 8), thus showing spasmolytic activity, mediated possibly through calcium channel blockade (CCB). Results of the study suggest that the spasmolytic effect of the plant extract is mediated through the presence of CCB-like constituent(s), which is concentrated in the n.-hexane fraction, and this study provides a strong mechanistic base for its traditional use in gastrointestinal disorders such as colic pain and diarrhea.
Antibacterial activity
In one study, the alcohol extract showed antibacterial activity against Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Staphylococcus citreus, Bacillus megaterium, Salmonella paratyphi. A and B, Salmonella marcescens, Proteus vulgaris,. and Shigella dysomei. (Vashi & Patel, Citation1987). The alcohol, ether, acetate buffer, and dilute sulfuric acid extract of the root showed antibacterial activity against Staphylococcus aureus. (Joshi & Magar, Citation1952). The extract of the rhizome showed moderate in vitro. antibacterial activity against Staphylococcus aureus, Streptococcus pyogenes, Streptococcus viridans, Diplococcus pneumoniae, Corynebacterium diphtheriae, Escherichia coli, Salmonella typhi, Salmonella paratyphi. A and B, and Shigella flexneri. (Vohora et al., Citation1989). The 80% ethanol extract showed inhibition of the two strains of Bacillus subtilis. and Staphylococcus aureus. using the agar diffusion method (Valsaraj et al., Citation1997).
The ether extract of AC in in vitro. studies using the disk diffusion method showed antibacterial activity against enteropathogenic Escherichia coli., Shigella sonnei., Salmonella typhi., Klebsiella pneumoniae., and Vibrio cholerae.. The extract showed antibacterial activity against Klebsiella pneumoniae. and Vibrio cholerae. using the tube dilution method (Vijaya & Ananthan, Citation1994). The essential oil from the rhizome showed a weak antibacterial activity against Staphylococcus aureus, Escherichia coli, Salmonella typhosum., and Shigella flexneri. (Chopra et al.,Citation1954). The volatile oil inhibited the growth of Mycobacterium tuberculosis. var. hominis. and Gram-negative organisms. The oil showed antibacterial activity against Staphylococcus albus, Corynebacterium diptheriae, Salmonella typhi, Salmonella faecalis, Bacillus pumilus, Streptococcus pyogenes., and Pseudomonas solanacearum. (Jain et al., Citation1974). The essential oil exhibited in vitro. antibacterial activity against Bacillus proteus, Escherichia coli, Staphylococcus pyogenes, Shigella shiga, Shigella boydii, Salmonella typhi,. and Salmonella paratyphi. (Alankararao & Rajendra Prasad, Citation1981; Kapil et al., Citation1983).
Antifungal activity
The essential oil showed antifungal activity against Aspergillus oryzae, A. nidulans, A. jumigatis, Penicillium aculeatum, Phomopsis destuctum., citrus decay pathogens Penicillium digitatum, P. italicum, Diplodia natalensis, Altemaria tenuis, Candida albicans, Epidermophyton cresens., and Microsporum gypseum. (Alankararao & Rajendra Prasad, Citation1981; Kapil et al., Citation1983). The alcohol extract possesses antifungal activity against Aspergillus niger, Penicillium selenium., and yeast Saccharomyces. (Vashi & Patel, Citation1987). The fungitoxicity data of the oil with those of its major constituents β.-asarone, asaraldehyde, acoradin (three asarone derivatives) against Helminthosporium oryzae. indicated that β.-asarone was the most active. β.-Asarone present was primarily responsible for its fungitoxicity (Saxena et al., Citation1990).
Antiviral activity
Badam (Citation1995) reported that the alcohol extract of the rhizome showed potent antiviral activity against Herpes simplex virus HSV-1 and HSV-2 at a concentration well below the cytotoxic concentration. Pretreatment of Vero cells with the extract did not inhibit viral replication of HSV-1 and HSV-2. It shows that host cells were not affected by the extract. β.-Asarone possesses strong inhibitory activity against the replication of both virus types. The crude alcohol extract and β.-asarone showed toxicity to the host cells.
Anthelmintic activity
The alcohol extract showed in vitro. anthelmintic activity against human Ascaris lumbricoides. (Kaleysa Raj, Citation1974). The essential oil inhibited the amplitude of rhythmic contractions of Ascaris lumbricoides. within 5 min of exposure. The oil produced partial paralysis of the movements; the phenolic and nonphenolic fractions of the same oil, when tested separately, caused complete paralysis within 25 and 5 min, respectively (Chaudhari et al., Citation1981). In one study, the essential oil showed nematicidal activity against Meloidogyne incognita. (Singh et al., Citation1991). Sharma et al. (Citation1985) performed a clinical trial on 147 children of age ranging from 5 to 11 years having round worm infestation; calamus powder 250 mg three times daily was given for -3 days. As a result, 83% were completely cured, whereas 17% remained unchanged.
Insecticidal activity
The essential oil showed insecticidal activity against houseflies Musca domestica. (Singh & Mehta, Citation1998). The oil inhibited the interstitial cell activity of instar larvae of Dysdercus koenigii.. β.-Asarone with its antigonadal function represented a new type of antigonadal agent that might afford a new and safe approach toward insect control (Saxena et al.,Citation1977). The essential oil showed antifeedant and growth inhibitory effects against third instar larvae of Spodoptera litura. when used as emulsified foliage sprays (Koul, Citation1987) and was also effective in controlling the stored grain insect Spodoptera litura. (Agarwal et al., Citation1973). The oil caused 100% mortality of Aedes aegypti. and Culex fatigans.. The oil vapors affected the hatching of eggs of all age groups of instar nymphs (Saxena & Srivastava, Citation1972). The oil vapors of the rhizomes caused sterility in male houseflies (Mathur & Saxena, Citation1975). The oil vapors also slowed morphologic changes in the ovaries of Thermobia domestica. (Saxena & Rohdendorf, Citation1974).
The solvent extracts and the volatile principle of the AC rhizome were found to be toxic to housefly Musca nebulo. and to Culex fatigans.. The powdered rhizome was found to be useful against bugs, moths, and lice (Subrahmanyam, Citation1949). The ethanolic extracts possessed the highest larvicidal activity against Culex fatigans. (Komalamisra et al., Citation2005). The aqueous suspension and cold alcohol extract showed insecticidal activity against plant insects Prodenia litura. and Dactynotus carthami.. The powder of the rhizome exhibited repellent activity against pulse beetle Callosobruchus chinensis. (Khan, Citation1986). The ether extract exhibited strong antifeeding, repellent, and insecticidal activities against the third instar larvae of sawfly Athalia proxima. (Pandey et al., Citation1977). The ethanol extract was found to have 100% mortality against the larvae of Ailanthus webworm Atteva fabriciella. (Ahmad et al., Citation1991).
Piscicidal activity
In one study, the crude extract of AC was screened for piscicidal activity against seven species of fishes found in the Doon valley. It showed strong piscicidal activity against seven species of fishes found in the Doon valley (Virdi, Citation1982)
Adulticidal activity
In one study, the hexane fraction from the methanol extract of AC rhizome was screened for its adulticidal activity with Standard WHO bioassay tests. The hexane fraction from the methanol extract of AC rhizome possessed the most effective adulticidal property, exhibiting LC50 and LC90 values of 0.04 mg/cm2 and 0.09 mg/cm2, respectively (Hidayatulfathi et al., Citation2004).
Diuretic activity
The ethanol (50%) extract of AC was screened for its diuretic activity in rats. The result of the study showed diuretic activity of AC (Aswal et al.,Citation1996).
Genotoxicity and Mutagenicity
AC is a mild co-carcinogen and may interfere with normal pregnancy inter-reactions. The effects of β.-asarone on chromosomes were studied in human lymphocyte cultures. A very strong effect on the induction of structural chromosome aberrations was found after metabolic activation and cellular damage occurred. The results demonstrate clearly the genotoxic potency of β.-asarone and suggested that only Acorus. with low content of β.-asarone should be used (Abel, Citation1987). α.-Asarone was mutagenic to Salmonella typhimurium. in a concentration-dependent fashion. α.-Asarone–induced mutagenicity required a promutagen mixture containing liver S-9 fraction and NADPH. The mutagenicity of α.-asarone was comparable with that induced by aflatoxin. Apparently, α.-asarone is a positive mutagen (Jin et al., Citation1982; Mukherjee & Wahile, Citation2006b). In another study, β.-asarone showed mutagenic activity in the Salmonella.-mammalian microsome assay, and the results of the study suggested that only commercial drugs free from or with a low content of β.-asarone should be used in human phytotherapy (Goeggelmann & Schimmer, Citation1983).
Conclusions
For ages, AC rhizome has been used for the treatment of various ailments in traditional and folklore medicine. The plant exhibits polyploidy, and the composition of the essential oil obtained from the plant rhizome depends on the karyotype. Of the phytoconstituents reported from Acorus. rhizomes, α.-asarone and β.-asarone are the predominant bioactive constituents. Various pharmacological activities of AC rhizome such as sedative, CNS depressant, behavior modifying, anticonvulsant, antispasmodic, cardiovascular, hypolipidemic, immunosuppressive, anti-inflammatory, cytoprotective, antioxidant, antidiarrheal, antimicrobial, anthelmintic, insecticidal, and diuretic have been reported by different workers. Together with this, some untoward effects of AC rhizome and its constituents α.-asarone and β.-asarone such as genotoxicity and mutagenicity have also been reported, which limits its therapeutic usage. Thus, this rhizome is well-known as a CNS active herb from Ayurvedic tradition and requires further research to establish the molecular mechanisms of evaluate its activity.
Acknowledgments
The authors wish to express their gratitude to the Department of Biotechnology (DBT), Government of India, for providing financial assistance through a research project to the School of Natural Product Studies, Jadavpur University. Thanks are also due to the Commonwealth Scholarship Commission, UK, for the Commonwealth Academic Staff Fellowship award to Dr. Pulok K. Mukherjee.
References
- Abel G (1987): Chromosome damage in human lymphocytes induced by β.-asarone. Planta Med 53: 251–253.
- Acuna UM, Atha DE, Ma J, Nee MH, Kennelly EJ (2002): Antioxidant capacities of ten edible North American plants. Phytother Res 16: 63–65.
- Ahmad M, Gupta BK, Bhandari RS (1991): Efficacy of some plant extracts against Atteva fabriciella. worm. Indian J Forestry 14: 5–7.
- Agarwal DC, Deshpande RS, Tipnis HP (1973): Insecticidal activity of Acorus calamus. on stored gram insects. Pesticides 7: 21.
- Agarwal SL, Dandiya PC, Singh KP, Arora RB (1956): A note on the preliminary studies of certain pharmacological actions of Acorus calamus L.. J Am Pharm Assoc 45: 655–656.
- Alankararao GSJG, Rajendra Prasad Y (1981): Antimicrobial property of Acorus calamus. Linn. in in vitro. studies. Indian Perfum 25: 4–6.
- Anonymous (2001): The Wealth of India A Dictionary of Indian Raw Materials & Industrial Products: First Supplement Series (Raw Materials). Vol. I. New Delhi, India, National Institute of Science Communication., pp 28–30.
- Arora RB (l965): Cardiovascular Pharmacotherapeutics of Six Medicinal Plants Indigenous to India. Award Monograph Series No.1. New Delhi, Hamdard National Foundation, pp. 421–450.
- Aswal BS, Goel AK, Kulshrestha DK, Mehrotra BN, Patnaik GK (1996): Screening of Indian plants for biological activity. Part XV. Indian J Exp Biol 34: 444–467.
- Badam L (1995): In vitro. studies on the effects of Acorus calamus. extract and β.-asarone on Herpes viruses. Deerghayu Intl 11: 16–18.
- Bains JS, Dhuna V, Singh J, Kamboj SS, Nijjar KK, Agrewala JN (2005): Novel lectins from rhizomes of two Acorus. species with mitogenic activity and inhibitory potential towards murine cancer cell lines. Int Immunopharmacol 5: 1470–1478.
- Bajpai A, Ojha JK, Sant HR (1995): Medicobotany of the Varanasi district, Uttar Pradesh, India. Int J Pharmacog 33: 172–176.
- Balaji Rao NS, Rajasekhar D, Chengal Raju D, Nagaraju N (1996): Ethno-medicinal notes on some plants of Tirumala hills for dental disorders. Ethnobotany 8: 88–91.
- Baxter RM, Dandiya PC, Kandel SI, Okany A, Walker GC (1960): Separations of hypnotic potentiating principles from the essential oil of Acorus calamus. Linn, of Indian origin by liquid gas chromatography. Nature 185: 466–467.
- Bhakuni DS, Goel AK, Jain S, Mehrotra BN, Patnaik GK, Prakash Y (1988): Screening of Indian plants for biological activity, Part XIII. Indian J Exp Biol 26: 883–904.
- Bhattacharya IC (1968): Effect of Acorus. (Vacha) oil on the amphetamine induced agitation, hexobarbital-sleeping time and on instrumental avoidance conditioning in rats. J Res in Indian Med 2: 195–201.
- Bist MK, Badoni AK (1990): Araceae in the folk life of the tribal populace in Garhwal Himalayas. J Eco Bot Phytochem 1: 21–24.
- Borthakur SK, Goswami N (1995): Herbal remedies from Dimoria of Kamrup district of Assam in Northeastern India. Fitoterapia 66: 333–340.
- Bose BC, Vijayvargiya R, Saiti AQ, Sharma SK (1960): Some aspects of chemical and pharmacological studies of Acorus calamus. Linn. J Am Pharm Assoc 49: 32–34.
- Cavazza G (1976): Les chimiotypes parmi les plantes aromatiques cas du calamus polyploide. Ann Fals Exp Chins 69: 833–844.
- Chak IM, Sharma JN (1965): Effect of asarone on experimentally induced contlict neurosis in rats. Indian J Exp Biol 3: 252–254.
- Chandra P (1980): A note on the preliminary study on Acorus calamus. L. in the treatment of bronchial asthma. J Res Ayur Siddha 1: 329–30.
- Chaudhari GN, Kobte CK, Nimbkar AY (1981): Search for anthelmintics of plant origin: Activities of volatile principles of Acorus calamus. against Ascaris lumbricoides.. Ancient Sci Life 1: 103–105.
- Chaudhury SS, Gautam SK, Handa KL (1957): Composition of calamus oil from calamus roots growing in Jammu and Kashmir. Indian J Pharm 19: 183–186.
- Chopra IC, Jamwal KS, Khajuria BN (1954): Pharmacological action of some common essential oil bearing plants used in indigenous medicine. Part I. Pharmacological action of Acorus calamus, Curcuma zedoaria, Xanthoxylum alatum. and Angelica archangelica.. Indian J Med Res 42: 381–384.
- Dandiya PC, Cullumbine H (1959): Studies on Acorus calamus. III. Some pharmacological actions of the volatile oil. J Pharmacol Exp Ther 125: 353–359.
- Dandiya PC, Baxter RM, Walker GC, Cullumbine H (1959a): Studies on Acorus calamus.. Part II. Investigation of volatile oil. J Pharm Pharmacol 11: 163–168.
- Dandiya PC, Cullumbine H, Sellers EA (1959b): Studies on Acorus calamus. IV Investigations on mechanism of action in mice. J Pharmacol Exp Ther 126: 334–337.
- Dandiya PC, Sharma JD (1962): Studies on Acorus calamus. Part V. Pharmacological actions of asarone and β.-asarone on central nervous system. Indian J Med Res 50:46–60.
- Dandiya PC, Menon MK (1963): Effect of asarone and β.-asarone on conditioned responses, fighting behaviour and convulsions. Br J Pharmacol 20: 436–442.
- Dandiya PC, Menon MK (1964): Actions of asarone on behaviour, stress and hyperpyrexia, and its interaction with central stimulants. J Pharmacol Exp Ther 145: 42–46.
- Dandiya PC, Menon MK (1965): Interaction of asarone with mescaline, amphetamine and tremorine. Life Sci 4: 1635–1641.
- Das PK, Malhotra CL, Dhalla NS (1962): Spasmolytic activity of asarone and essential oil of Acorus calamus. Linn. Arch Int Pharmacodyn Ther 135: 167–177.
- Dasgupta SR, Patra BB, Sikdar S (1977): Preliminary studies of the effect of a chloroform extracted factor from Acorus calamus. on the behaviour of conscious rhesus monkeys. Sci Cult 43: 218–219.
- Dhalla NS, Bhattacharya IS (1968): Further studies on neuropharmacological actions of Acorus. oil. Arch Int Pharmacodyn Ther 172: 356–365.
- Dhalla NS, Malhotra CL, Sastry MS (1961): Effect of Acorus. oil in vitro. on the respiration of rat brain. J Pharm Sci 50: 580–582.
- Dobriyal RM, Singh GS, Rao KS, Saxena KG (1997): Medicinal plant resources in Chhakinal watershed in the Northwestern Himalaya. J Herb Spices Med Plant 5: 15–27.
- Garduno L, Salazar M, Salazar S, Morelos ME, Labarrios F, Tamariz J, Chamorro GA (1997): Hypolipidaemic activity of α.-asarone in mice. J Ethnopharmacol 55: 161–163.
- Gilani AU, Shah AJ, Ahmad M, Shaheen F (2006). Antispasmodic effect of Acorus calamus. Linn. is mediated through calcium channel blockade. Phytother Res 20: 1080–1084.
- Goeggelmann W, Schimmer O (1983): Mutagenicity testing of β.-asarone and commercial calamus drugs with Salmonella typhimurium.. Mutat Res 121: 191–194.
- Govindarajan R, Agnihotri AK, Khatoon S, Rawat AKS, Mehrotra S (2003): Pharmacognostical evaluation of an antioxidant plant—Acorus calamus.. Nat Prod Sci 9: 264–269.
- Hazra R, Guha D (2003): Effect of chronic administration of Acorus calamus. on electrical activity and regional monoamine levels in rat brain. Biogenic Amines 17: 161–169.
- Hidayatulfathi O, Sallehuddin S, Ibrahim J (2004): Adulticidal activity of some Malaysian plant extracts against Aedes aegypti. Linnaeus. Trop Biomed 21: 61–67.
- Houghton PJ, Mukherjee PK, Kumar V, Mal M (2006a): Acetylcholinesterase inhibition of oil from Acorus calamus. rhizome. Planta Med 72: 996.
- Houghton PJ, Kumar V, Govindarajan R, Mukherjee PK (2006b): Asarones in Acorus calamus. and their acetylcholinesterase inhibition. J Pharm Pharmacol Supplement 1: A-55.
- Howes MR, Houghton PJ (2003): Plants used in Chinese and Indian traditional medicine for improvement of memory and cognitive function. Pharmacol Biochem Behav 75: 513–527.
- Jain N, Suri RK (1980): Insecticidal, insect repellent and piscicidal plants of Dehradun. Nagarjun 23: 177–181.
- Jain SP (1989): Tribal remedies from Saranda forest Bihar, India-I. Intl J Crude Drug Res 27: 29–32.
- Jain SP, Puri HS (1994): An ethno-medico-botanical survey of Parbati valley in Himachal Pradesh, India. J Econ Tax Bot 18: 321–327.
- Jain SR, Jain PR, Jain MR (1974): Antibacterial evaluation of some indigenous volatile oils. Planta Med 26: 196–199.
- Jha RR, Varma SK (1996): Ethnobotany of Sauria Pahanas of Santhal Pargana, Bihar: 1. Medicinal plants. Ethnobotany 8: 31–35.
- Jin Z, Qin W, Chen X (1982): Mutagenicity of α.–asarone from Acorus calamus. and its metabolic pathway in vitro.. Zhejiang Yike Daxue Xuebao 11: 1–3.
- Joshi CG, Magar NG (1952): Antibiotic activity of some Indian medicinal plants. J Sci Ind Res 11B: 261–263.
- Kaleysa Raj R (1974): Screening of some indigenous plants for anthelmintic action against human Ascaris lumbricoides.. Indian J Physiol Pharmacol 18: 129–131.
- Kapil VB, Sinha AK, Sinha GK (1983): Antibacterial and antifungal study of some essential oils and their constituents from the plants of Kumaon and its Tarai tract. Bull Med Ethnobot Res 4: 124–129.
- Kapur SK (1993): Ethno-medico plants of Kangra valley (Himachal Pradesh). J Econ Tax Bot 17: 395–408.
- Khan MI (1986): Efficacy of Acorus calamus. L. rhizome powder against pulse beetle (Callosobruchus chinensis. L.). Pakistan Vet Res J10: 72.
- Khare AK, Sharma MK (1982): Experimemal evaluation of antiepileptic activity of Acorus. oil. J Sci Res Plant Med 3: 100–103.
- Kirtikar KR, Basu BD (1954): Indian Medicinal Plants. Vol. III. Allahabad, India, Basu LM, pp 2045–2048.
- Kirtikar KR, Basu BD (1987): Indian Medicinal Plants. Vol. IV. Dehradun, India, M/s Bishen Sing, Mahendra Pal Sing Publishers, pp 1229–1230.
- Komalamisra N, Trongtokit Y, Rongsriyam Y, Apiwathnasorn C (2005): Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian J Trop Med Public Health 36: 1412–1422.
- Koul O (1987): Antifeedant and growth inhibitory effects of calamus oil and neem oil on Spodoptera litura. under laboratory conditions. Phytoparasitica 15: 169–180.
- Madan BR, Arora RB, Kapila K (1960): Amiconvulsant, antiveratrinic and antiarrhythmic actions of Acorus calamus. Linn. an Indian indigenous drug. Arch Intl Pharmacodyn Ther 124: 201–211.
- Malhotra CL, Prasad K, Dhalla NS, Das PK (1961): Effect of hersaponin and Acorus. oil on noradrenaline and 5-hydroxytryptamine content of rat brain. J Pharm Pharmacol 13: 447–448.
- Malhotra CL, Das PK, Dhalla NS (1962): Investigations on the mechanism of potentiation of barbiturate hypnosis by hersaponin, Acorus. oil, reserpine and chlorpromazine. Arch Intl Pharmarodyn Ther 138: 537–547.
- Mamgain P, Singh RH (1994): Controlled clinical trial of the lekhaniya drug vaca. (Acorus calamus) in cases of Ischaemic heart diseases. J Res Ayur Siddha 15: 35–51.
- Manikandan S, Devi RS (2005): Antioxidant property of β.-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res 52: 467–474.
- Manikandan S, Srikumar R, Jeya Parthasarathy N, Sheela Devi R (2005). Protective effect of Acorus calamus. Linn on free radical scavengers and lipid peroxidation in discrete regions of brain against noise stress exposed rat. Biol Pharm Bull 28: 2327–2330.
- Manis G, Rao A, Karanth KS (1991): Neuropharmacological activity of Acorus calamus.. Fitoterapia 62: 131–137.
- Mao AA (1993): A preliminary report on the folklore botany of Mao Nagas of Manipur (India). Ethnobotany 5: 143–147.
- Mathur AC, Saxena BP (1975): Induction of sterility in male houseflies by vapours of Acorus calamus. L oil. Naturwissenschaften 62: 576–577.
- Megoneitso Rao RR (1983): Ethnobotanical studies in Nagaland–4. Sixty two medicinal plants used by the Angami-Nagas. J Econ Tax Bot 4: 167–172.
- Mehrotra S, Mishra KP, Maurya R, Srimal RC, Yadav VS, Pandey R, Singh VK (2003): Anticellular and immunosuppressive properties of ethanolic extract of Acorus calamus. rhizome. Intl Immunopharmacol 3: 53–61.
- Menon MK, Dandiya PC (1967): The mechanism of the tranquillizing action of asarone from Acorus calamus.Linn. J Pharm Pharmacol 19: 170–175.
- Mohanty RB, Padhy SN, Dash SK (1996): Traditional phytotherapy for diarrhoeal diseases in Ganjan and Phulbani district of South Orissa, India. Ethnobotany 8: 60–65.
- Moholkar AL, Majumdar SM, Pandit PR, Joglekar GY (1975): Role of potassium in pharmacological activity of 50% alcoholic extract of Rubia cordifolia, Acorus calamus. and Withania somnifera.. J Res Indian Med 10: 34–38.
- Mukherjee PK (2002): Quality Control of Herbal Drugs–An Approach to Evaluation of Botanicals. New Delhi, India, Business Horizons, pp 692–694.
- Mukherjee PK (2003): Plant products with hypocholesterolemic potentials. Adv Food Nutr Res 47: 278–324.
- Mukherjee PK, Wahile A (2006a): Integrated approaches towards drug development from ayurveda and other Indian system of medicines. J Ethnopharmacol 103: 25–35.
- Mukherjee PK, Wahile A (2006b): Perspectives of safety for natural health products. In: Sharma RK, Arora A, eds., Herbal Drugs–A Twenty first Century Perspective, New Delhi, India, Jaypee Brothers Medicinal Publishers Ltd., pp. 50–59.
- Nawamaki K, Kuroyanagi M (1996): Sesquiterpenoids from Acorus calamus. as germination inhibitors. Phytochemistry 43: 1175–1182.
- Negi GS, Joshi BC (1981): Chemical analysis of some medicinal plants of Garhwal region. Himalayan. J Sci 1:60–62.
- Nigam MC, Ahmad A, Misra LN (1990): GC-MS examination of the essential oil of Acorus calamus.. Indian Perfum 34: 282–285.
- Nishiyama N, Zhou Y, Saito H (1994): Ameliorative effects of chronic treatment using DX 9386, a traditional Chinese prescription on learning performance and lipid peroxide content in senescence accelerated mouse. Biol Pharm Bull 17: 1481–1484.
- Oh MH, Houghton PJ, Whang WK, Cho JH (2004): Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 11: 544–548.
- Oprean R, Oprean L, Tamas M, Sandulescu R, Roman L (2001): Essential oils analysis. II. Mass spectra identification of terpene and phenylpropane derivatives. J Pharm Biomed Anal 24: 1163–1168.
- Pandey ND, Singh M, Tewari GC (1977): Antifeeding repellent and insecticidal properties of some indigenous plant materials against mustard sawfly (Athalia proxima.) Klug. Indian J Entomol 39: 60–64.
- Parab RS, Mengi SA (2002): Hypolipidemic activity of Acorus calamus. L. in rats. Fitoterapia 73: 451–455.
- Patra A, Mitra AK (1981): Constituents of Acorus calamus.: Structure of acoramone: carbon-13-NMR spectra of cis-and trans-.asarone. J Nat Prod 44: 668–669.
- Rafatullah S, Tariq M, Mossa JS, Al Yahya MA, Al Said MS, Ageel AM (1994): Antisecretory, antiulcer and cytoprotective properties of Acorus calamus. in rats. Fitoterapia 65: 19–23.
- Rai R, Siddiqui IR, Singh J (1998): Triterpenoid saponins from Acorus calamus.. Indian J Chem 378: 473–476.
- Raina VK, Srivastava SK, Syamasunder KV (2003): Essential oil composition of Acorus calamus. L. from the lower region of the Himalayas. Flavour Fragr J 18: 18–20.
- Rajasekharan S, Srivastava TN (1977): Ethnobotanical study on vacha. and a preliminary clinical trial on bronchial asthma. J Res Indian Med Yoga Homoeop 12: 92–96.
- Saxena BP, Srivastava JB (1972): Effect of Acorus calamus. L. oil vapours on Dysdercus koenigii. F. Indian J Exp Biol 10: 391–393.
- Saxena BP, Rohdendorf EB (1974): Morphological changes in Thermubia domestica. under the influence of Acorus calamus. oil vapours. Experientia 30: 1298–1300.
- Saxena BP, Kaul O, Tikku K, Atal CK (1977): A new insect chemosterilant isolated from Acorus calamus. L. Nature 270: 512–513.
- Saxena DB (1986): Phenyl indane from Acorus calamus.. Phytochemistry 25: 553–555.
- Saxena DB, Tomar SS, Singh RP (1990): Fungitoxicity of chemical components and some derivatives from Anethum sowa. and Acorus calamus.. Indian Perfum 34: 199–203.
- Shipochliev T (1968): Pharmacological study of a group of essential oils II. Effect of essential oils on the motor activity and general state of mice in separate applications or after iproniazid phosphate. Veterinarno Meditsinski Nauki 5: 87–92.
- Singh D, Mehta S (1998): Screening of plant materials for repellent and insecticidal properties against pulse beetle (Callosobruchus chinensis.) and housefly (Musca domestica.). J Med Aromat Plant Sci 20: 397–400.
- Singh KK, Prakash A (1994): Indigenous phytotherapy among the Gond tribes of Uttar Pradesh, India. Ethnobotany 6: 37–41.
- Singh M (1989): Acorus calamus. an Indian medicinal plant: Its effect on escape/avoidance learning and general activity in albino rats. J Sci Res Plant Med 10: 34–45.
- Singh RP, Tomar SS, Devakumar C, Goswami BK, Saxena DB (1991): Nematicidal efficacy of some essential oils aganinst Meloidogyne incognita.. Indian Perfum 35: 35–37.
- Singh V (1995): Traditional remedies to treat asthma in North West and trans-Himalayan region in Jammu and Kashmir state. Fitoterapia 66:507–509.
- Singh VK, Ali ZA, Zaidi STH, Siddiqui MK (1996): Ethnomedicinal uses of plants from Gonda district forests of Uttar Pradesh, India. Fitoterapia 67: 129–139.
- Sharma JD, Dandiya PC, Baxter RM, Kandel SI (1961): Pharmacodynamical effects of asarone and β.-asarone. Nature 192: 1299–1300.
- Sharma PK, Dandiya PC (1969): Synthesis and some pharmacological actions of asarone. Indian J Appl Chem 32: 236–238.
- Sharma RD, Chaturvedi C, Tewari PV (1985): Helminthiasis in children and its treatment with indigenous drugs. Ancient Sci Life 4: 245–257.
- Sharma RN, Deshpande SG, Tungikar VB, Joseph M (1994): Toxicity of natural essential oils to mosquitoes Aedes aegypti. and Culex fatigans.. Geobios 21: 162–165.
- Shoba FG, Thomas M (2001): Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J Ethnopharmacol 76: 73–76.
- Shukla PK, Khanna VK, Ali MM, Maurya RR, Handa SS, Srimal RC (2002): Protective effect of Acorus calamus. against acrylamide induced neurotoxicity. Phytother Res 16: 256–260.
- Shukla PK, Khanna VK, Ali MM, Maurya R, Khan MY, Srimal RC (2006): Neuroprotective effect of Acorus calamus. against middle cerebral artery occlusion-induced ischaemia in rat. Hum Exp Toxico l25: 187–194.
- Srivastava TN, Rajasekharan S, Badola DP, Shah DC (1986): An index of the available medicinal plants used in Indian system of medicine from Jammu and Kashmir state. Ancient Sci Life 6: 49–63.
- Srivastava VK, Singh BM, Negi KS, Pant KC, Sunjea P (1997): Gas chromatographic examination of some aromatic plants of Uttar Pradesh hills. Indian Perfum 41: 129–139.
- Subrahmanyam TV (1949): Sweet flag (Acorus calamus.) a potential source of valuable insecticide. J Bombay Nat Hist Soc 48: 338–341.
- Tewari IC, Sanwal P, Singh J, Joshi P (1984): Preliminary phytochemical screening of medicinal plants of hilly districts (Kumaon and Garhwal divisions) of UP. Bull Med Ethnobot Res 5: 71–81.
- Tripathi AK, Singh RH (1995): Clinical study on an indigenous drug vaca. (Acorus calamus.) in the treatment of depressive illness. J Res Ayur Siddha 16: 24.
- Valsaraj R, Pushpangadan P, Smitt UW, Adseren A, Nymaan U (1997): Antimicrobial screening of selected medicinal plants from India. J Ethnopharmacol 58: 75–83.
- Varde AB, Ainapure SS, Naik SR, Amladi SR (1988): Anti–inflammatory activity of coconut oil extract of Acorus calamus, Ocimum sanctum. and Ocimum basilicum. in rats. Indian Drugs 25: 226–228.
- Vashi IG, Patel HC (1987): Chemical constituents and antimicrobial activity of Acorus calamus. Linn. Comp Physiol Eco 12: 49–51.
- Vashist VN, Handa KL (1964): Scientific background of havan and incenses. Indian Oil and Soap J 30: 130–135.
- Vijaya K, Ananthan S (1994): Effect of three medicinal plants on enteropathogens an in vitro. study. Biomedicine 14: 21–23.
- Virdi GS (1982): A study on the piscicidal properties of Acorus calamus. L., Sapindus mukorossi. Benth. and Xeromphis spinosa. Keay upon seven species of fishes of North India. Indian J Physiol Nat Sci 2: 2–8.
- Viswanathan K (1995): Survey on medicinal spices of the Nilgiris. Ancient Sci Life 14: 258–267.
- Vohora SB, Shah SA, Dandiya PC (1990): Central nervous system studies on an ethanol extract of Acorus calamus. rhizomes. J Ethnopharmacol 28: 53–62.
- Vohora SB, Shah SA, Sharma K, Naqvi SAH, Dandiya PC (1989): Antibacterial, antipyretic, analgesic and antiintlammatory studies on Acorus calamus. Linn. Ann Nat Acad Med Sci 25: 13–20.
- Yamamura S, Iguchi M, Nishiyama A, Niwa M, Koyama H, Hirata Y (1971): Sesquiterpenes from Acorus calamus. L. Tetrahedron 27: 5419–5431.
- Zanoli P, Avallone R, Baraldi M (1998): Sedative and hypothermic effects induced by β.-asarone, a main component of Acorus calamus.. Phytother Res 12: 114–116.
- Zhang Y, Takashina K, Saito H, Nishiyama N (1994): Anti-aging effect of DX-9386 in senescence accelerated mouse. Biol Pharm Bull 17: 866–868.