Abstract
The current study investigated the effect of 70% acetone extract of Punica granatum. L. (Punicaceae; pomegranate family) fruits on hepatic marker enzymes, antioxidants, and tissue peroxidative damage during isoniazid- and rifampicin-induced hepatotoxicity. Isoniazid and rifampicin (each at doses of 50 mg/kg body weight, intraperitoneally) for 15 days caused liver injury in rats that was manifested by significant elevation in the level of lipid peroxides, serum hepatic marker enzymes (glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, lactate dehydrogenase, alkaline phosphatase), and by a significant decrease in the enzymic antioxidants (superoxide dismutase, catalase, glutathione S.-transferase, glutathione peroxidase) and nonenzymic antioxidants (reduced glutathione, vitamin C, and vitamin E). Cotreatment with 70% acetone extract of Punica granatum. fruits significantly prevented these alterations and restored the enzyme activities and lipid peroxides to near normalcy. These findings demonstrate the hepatoprotective potential of the acetone extract of Punica granatum. fruits on tissue defense systems during isoniazid- and rifampicin-induced hepatotoxicity in rats.
Introduction
The incidence of tuberculosis is increasing worldwide (Shingadia & Novelli, Citation2003). Isoniazid and rifampicin are the most common antibiotics used for the effective treatment of tuberculosis (Stork & Hoffman, Citation1996). Although the efficacy of isoniazid and rifampicin had been proved beyond doubt, there has been a steady increase in the documentation of serious side effects during chronic use of these drugs that can cause significant morbidity, and quite often one of the regimens has to be discontinued (Dubey et al., Citation1985).
The great susceptibility of liver damage to chemical agents is a consequence of its primary role in the metabolism of foreign substances (Hussain et al., Citation2003). Hepatotoxicity associated with hepatitis is the major adverse effect of isoniazid and rifampicin (Lee, Citation1995). The toxic effect of isoniazid was reported to result from conversion of monoacetyl hydrazine (formed by the hydrolysis of acetylisoniazid) to a toxic metabolite via microsomal enzyme Cyt-P4502E1 (Watkins, Citation1990; Schwab et al., Citation1988). A potent acylating agent formed from monoacetyl hydrazine binds covalently to apparently vital hepatic macromolecules and causes hepatic necrosis (Mitchell et al., Citation1975). Rifampicin is a known microsomal enzyme (P-4502E1) inducer and increases the concentration of toxic metabolites of isoniazid (Yamamoto et al., Citation1986). Albano et al. (Citation1985) demonstrated the formation of free-radical intermediates during isoniazid metabolism by Cyt-P450. The metabolism of the drugs by phase I drug-metabolizing enzymes is often accompanied by the generation of oxygen free radicals, which in turn initiates lipid peroxidation leading to oxidative stress (Coon et al., Citation1992). Because oxidative stress due to free radicals appears to be the prime cause in the genesis of liver damage, any natural compound with antioxidant properties that might contribute toward alleviation of damage might have a significant role in maintaining health when used as a medicine or consumed as a part of normal diet.
In recent years, more attention has been paid to the antioxidants contained in fruits because epidemiologic studies revealed that high fruit intake was associated with reduced mortality and morbidity of cardiovascular disease and some types of cancer, and one of the possible mechanisms was attributed to the antioxidant activity presented by the fruits. Punica granatum. L. (Punicaceae), commonly known as pomegranate, is described for its medicinal properties in Ayurveda (Ross et al., Citation2001). It has long been esteemed as food and medicine and as a diet to convalesce after diarrhea. It has been reported to be anthelmintic (Singhal, Citation1983), antidiabetic, antidiarrheal (Jafri et al., Citation2000), antibacterial (Prashanth et al., Citation2001), antimicrobial (Anesini & Perez, Citation1993), antifungal (Charya et al., Citation1979) and antiatherogenic (Kaplan et al., Citation2001). Analyses of fruits demonstrated that Punica granatum. contained very high concentration of antioxidants, that is, 11.33 mmol/100 g (Halvorsen et al., Citation2002). The active constituents present in Punica granatum. include polyphenolic flavonoids (catechins, ellagic tannins, ellagic acid) and anthocyanidins (delphinidin, cyanidin, and pelargonidin) (Aviram et al., Citation2000). The antioxidant potential of 70% acetone extract of Punica granatum. fruits and its anthocyanidin contents have already been established (Noda et al., Citation2002).
As the antihepatotoxic potential of Punica granatum. has not been studied so far, the current investigation was undertaken to study the effect of 70% acetone extract of Punica granatum. fruits in reducing hepatotoxicity and associated oxidative stress induced by isoniazid and rifampicin.
Materials and Methods
Chemicals
Isoniazid and rifampicin were procured from Lupin Ltd. (Aurangabad, India). All other chemicals used were of analytical grade.
Preparation of extract
The fresh fruits of Punica granatum. were obtained in the month of June 2005 from Koyambedu Fruit Market in Chennai. The fruits were authenticated by Dr. Ramani Bai, Professor, Centre for Advanced Studies in Botany, University of Madras (Chennai, India). The fruits were peeled, and its edible portion (seedcoats and juice) was squeezed in 70% acetone–30% distilled water (1:20, by w/v). The red extract was filtered through filter paper (Whatman no. 1). The filtrate was freeze-dried. The freeze-dried extract was stored at 4°C for further use (Noda et al., Citation2002).
Animals
Adult male albino rats of Wistar strain weighing about 120–150 g were obtained from the Tamilnadu Veterinary and Animal Science University (Chennai, India). They were acclimatized to animal house conditions and fed commercial pellet rat chow (Hindustan Lever Ltd., Bangalore, India) and water ad libitum.. This study was conducted according to the ethical norms approved by the Ministry of Social Justices and Empowerment, Government of India, and the animal ethics committee guidelines of our institution.
Experimental design
The rats were divided into four groups with six animals in each group as follows: group 1, control; group 2, isoniazid and rifampicin administered; group 3, Punica granatum. extract administered; group 4, Punica granatum. treated plus antituberculosis drugs administered. Drug administration was as follows. Punica granatum. extract was given orally (400 mg/kg b.w.) through an intragastric feeding tube over a period of 15 days along with isoniazid and rifampicin. The particular dosage was fixed after trying out different doses (100, 200, 300, 400, 500, and 600 mg/kg b.w.) in rats. The drug showed protection in a dose-dependent manner. As the dosage of 400 mg/kg body weight showed maximum hepatoprotective effect, this particular dosage was fixed as the optimum dosage for the study. Isoniazid and rifampicin, each at the doses of 50 mg/kg body weight, were injected intraperitoneally for 15 days to induce hepatotoxicity in rats (Prabakan et al., Citation2000).
Biochemical assays
The activities of diagnostic marker enzymes, namely, glutamate oxaloacetate transaminase, glutamate pyruvate transaminase (Bergmeyer & Bernt, Citation1974), lactate dehydrogenase (King, Citation1965), and alkaline phosphatase (King & Armstrong, Citation1934), were assayed in serum. Further, the levels of antioxidants, namely, catalase (Takahara & Hamilton, Citation1960), superoxide dismutase (Misra & Fridovich, Citation1972), and glutathione-dependent enzymes, glutathione S.-transferase (Habig et al., Citation1974) and glutathione peroxidase (Rotruck et al., Citation1973) were determined. Total reduced glutathione (Ellman, Citation1959), levels of lipid peroxides (Ohkawa et al., Citation1979), levels of vitamins C (Omaye et al., Citation1979) and E (Desai, Citation1984) were estimated in the liver of experimental group of rats.
Statistical analysis
Results were expressed as mean ±SD for six animals in each group. All the grouped data were statistically evaluated with SPSS/10 software. Hypothesis testing methods included one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test. p values of less than 0.05 were considered to indicate statistical significance.
Results
Hepatic marker enzymes
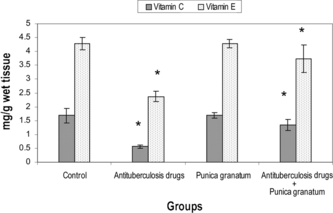
shows the activities of marker enzymes such as Glutamate oxaloacetate transaminase (GOT), Glutamate pyruvate transaminase (GPT), Lactate dehydrogenase (LDH), and Alkaline phosphatase (ALP) in the serum of control and experimental groups of rats. Marked elevation (p < 0.05) in the activities of these enzymes were observed in group 2 (antitubercular drugs administered rats) when compared with group 1 (control rats). Activities of these enzymes in serum were maintained at near normal (p < 0.05) levels in the group 4 rats cotreated with 70% acetone extract of Punica granatum.. Group 3, rats treated with Punica granatum. extract alone, did not show any changes when compared with group 1 (control rats), which confirms the nontoxic nature of the 70% acetone extract of Punica granatum..
Table 1.. Effect of Punica granatum. on the activities of marker enzymes in serum of control and experimental groups of rats.
Hepatic antioxidants
presents the activities of Superoxide dismutase (SOD), Catalase (CAT), Glutathione-S-transferase (GST), and Glutathione peroxidase (GPx) in the liver of control and experimental groups of rats. presents the level of GSH and shows the levels of vitamins C and E in the liver of control and experimental groups of rats. A significant (p < 0.05) decrease was observed in GSH, vitamins C and E levels, and in the activities of antioxidant enzymes GPx, GST, SOD, and CAT (p < 0.05) in group 2 (antituberculosis drugs administered rats). Cotreatment with Punica granatum. extract (group 4) significantly prevented (p < 0.05) these alterations when compared with group 2 (antitubercular drugs administered rats).
Table 2.. Effect of Punica granatum. on the activities of antioxidant enzymes in the livers of control and experimental groups of rats.
Figure 1 Level of glutathione in the livers of control and experimental groups of rats. Results are expressed as mean ± SD (n = 6). *p < 0.05. Comparisons are made between group 1 (control) with group 2 (antituberculosis drugs induced) and group 2 with group 4 (Punica granatum. + antituberculosis drugs).
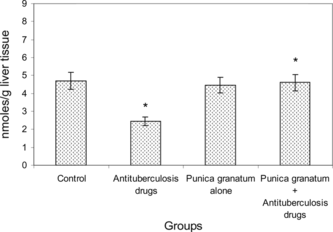
Figure 2 Levels of vitamins C and E in the livers of control and experimental groups of rats. Results are expressed as mean ± SD (n = 6). *p < 0.05. Comparisons are made between group 1 (control) with group 2 (antituberculosis drugs induced) and group 2 with group 4 (Punica granatum. + antituberculosis drugs).
Hepatic lipid peroxides
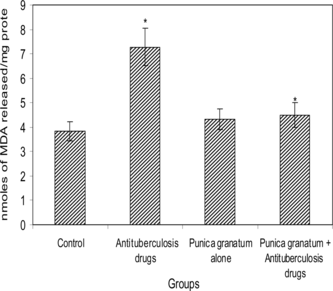
indicates the level of lipid peroxides (LPO) in the liver of control and experimental groups of rats. Maximum induction of lipid peroxidation was observed in group 2 rats when compared with group 1 rats. The altered metabolic changes were significantly (p < 0.05) restored to near normal levels in the group 4 rats treated with Punica granatum. extracts.
Figure 3 Levels of lipid peroxides in the livers of control and experimental groups of rats. Results are expressed as mean ± SD (n = 6). *p < 0.05. Comparisons are made between group 1 (control) with group 2 (antituberculosis drugs induced) and group 2 with group 4 (Punica granatum. + antituberculosis drugs).
Discussion
An increase in the incidence rate of drug-induced hepatitis due to antituberculosis chemotherapy has been reported. Side effects of the most commonly used first-line antituberculosis drugs range from minor gastrointestinal symptoms to severe hepatotoxicity (Lee, Citation1995). It appears that several forms of hepatic damage may be caused in part by oxidative stress, a condition caused by the formation of reactive oxygen species (Arthur, Citation1988; Hoek & Pastorino, Citation2002; Kaplowitz, Citation2002).
Glutamate oxaloacetate transaminase, glutamate pyruvate transaminase, lactate dehydrogenase, and alkaline phosphatase are the most sensitive markers that are considered as indices for the diagnosis of the liver diseases. In the current study, marked elevations in the activities of these enzymes were observed in the serum of isoniazid- and rifampicin-treated rats. Increased activities of these marker enzymes in the serum are indicative of cellular damage and loss of functional integrity of cell membrane (Bhakta et al., Citation1999). An acetone extract (70%) of Punica granatum. fruits seems to preserve the structural integrity of the hepatocellular membrane as evident from the significant reduction in these enzymes in rats administered antituberculosis drugs.
Glutathione peroxidase, glutathione S.-transferase, catalase, and superoxide dismutase are the enzymic antioxidants that form the first line of cellular defense against oxidative injury. Alterations in the levels of circulating antioxidants and intracellular scavenging enzymes that indicate a strong imbalance of cellular defense system occur during increased peroxidative processes. Catalase catalyzes the degradation of hydrogen peroxide to water. Superoxide dismutase converts superoxide radicals to hydrogen peroxide and molecular oxygen. They protect the cellular constituents against oxidative damage. A significant decrease in the activities of superoxide dismutase and catalase were observed in the rats treated with antituberculosis drugs, which may be due to the interaction of the accumulated free radicals with the associated metal ions or with the amino acids present in the active site of these enzymes (Fee & Briggs, Citation1975). This effect was reversed in the rats cotreated with 70% acetone extract of Punica granatum. fruits, which suggests that the extract may have the ability to prevent the deleterious effects induced by free radicals. Moreover, Punica granatum. has also been reported to be a quencher of free radicals (Hasten, Citation1983; Schubert et al., Citation1999).
A significant decrease in the level of reduced glutathione observed in the rats treated with antitubercular drugs may be due to its increased utilization during the burst of reactive oxygen species production in protecting “SH” group containing proteins from lipid peroxides (Halliwell, Citation1990). Cotreatment with 70% acetone extract of Punica granatum. fruits in antitubercular drugs–treated rats showed a tendency for the restoration of altered reduced glutathione level toward near-normal, which shows that the extract tends to prevent the tissue depletion of reduced glutathione.
Glutathione S.-transferase defends cells against a wide variety of toxic insults from chemicals, metabolites, and oxidative stress (Singh et al., Citation2002). Glutathione peroxidase, a selenium-containing enzyme, oxidizes the reduced glutathione and helps to dispose accumulated hydrogen peroxide and reduces lipid peroxides in the cells (Flohe, Citation1982). Significant decrease in the activities of glutathione S.-transferase and glutathione peroxidase in rats administered antitubercular drugs may be due to the depletion of reduced glutathione levels, thereby resulting in accumulation of hydrogen peroxide. Cotreatment with 70% acetone extract of Punica granatum. fruits restored the levels of glutathione S.-transferase and glutathione peroxidase to near normal, which shows the antioxidant potential of the extract against oxidative damage.
Lipid peroxidation is considered as a critical mechanism of tissue damage occurring during hepatic failure (Comporti, Citation1985). Inactivation of antioxidant defense during oxidative damage leads to an increase in membrane lipid peroxidation and subsequent hemolysis (Chiu et al., Citation1982). Measurement of the end products of lipid peroxidation, for example, malondialdehyde, is used by many investigators to estimate in vivo. or in vitro. damage to lipids (Mahle & Dasgupta, Citation1997). A significant increase in the levels of malondialdehyde in terms of ‘thiobarbituric acid reactive substances’ indicating enhanced lipid peroxidation was observed in rats administered antitubercular drugs. Isoniazid and rifampicin appear to cause an imbalance in the antioxidant defense system by depressing the reduced glutathione levels and thereby inhibiting reduced glutathione–dependent enzymes, which enhances the free radical–mediated peroxidation of lipids. Cotreatment with 70% acetone extract of Punica granatum. fruits causes a significant decrease in the levels of thiobarbituric acid reactive substances in rats treated with isoniazid and rifampicin, which may be attributed to the anti–lipid peroxidative activity of Punica granatum. that protects the liver from lipid peroxidation (Kaplan et al., Citation2001).
Vitamin C has been demonstrated to be an efficient antioxidant. It can act both directly, by reaction with aqueous peroxyl radicals, and indirectly, by restoring the antioxidant properties of vitamin E. Vitamin E is a principal lipid-soluble antioxidant in cell membranes that protects critical cellular structures against oxidative damage (Ravikumar et al., Citation2005). A significant decrease in the levels of vitamins C and E in isoniazid- and rifampicin-treated rats may be due to the excessive utilization of antioxidants employed in quenching the free radicals produced during liver cell injury. The improvement in the levels of nonenzymic antioxidants in Punica granatum.–treated rats suggests that the 70% acetone extract of Punica granatum. fruits may have the ability to normalize the imbalance induced by isoniazid and rifampicin in the hepatic cellular levels of vitamins C and E and of reduced glutathione. Reduced glutathione might maintain the vitamin E levels either by direct reduction of α-tocopheroyl radical to vitamin E or via the reductive mode of vitamin C (Ip & Ko, Citation1996).
The protection conferred by Punica granatum. may be due to its antiperoxidative activity (Kaplan et al., Citation2001), ability to quench free radicals (Schubert et al., Citation1999), and ability to chelate metal ions (Aviram et al., Citation2000). Thus, acetone extract of Punica granatum. fruits retains the activities of antioxidant enzymes and protects the liver cells against isoniazid- and rifampicin-induced damage. The protection offered by 70% acetone extract of Punica granatum. fruits may be attributed to the presence of active components such as anthocyanidins (delphinidin, cyaniding, and pelargonidin), the antioxidant properties of which are well documented (Noda at al., Citation2002).
Conclusions
It can be concluded from the above observations that the acetone extract of Punica granatum. fruits offers protection to the liver and significantly counteracts the oxidative stress by reduction of lipid peroxidation. Punica granatum. inactivates free radicals and increases the antioxidant levels, which constitutes the foremost defense system that limits the toxicity associated with free radicals formed during isoniazid- and rifampicin-induced hepatic injury.
Acknowledgment
The financial assistance from Birla Smarak Kosh in the form of Junior Research Fellowship is acknowledged with thanks.
References
- Albano E, Tomasi A, Vannini V, Dlanzani MU (1985): Detection of free radical intermediates during isoniazid and iproniazid metabolism by isolated rat hepatocytes. Biochem Pharmacol 24: 381–382.
- Anesini C, Perez C (1993): Screening of plants used in Argentine folk medicine for antimicrobial activity. J Ethnopharmacol 39: 119–128.
- Arthur MJP (1988): Reactive oxygen intermediates and liver injury. J Hepatol 6: 125–131.
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, Hayek T, Presser D, Fuhrman B (2000): Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr 71: 1062–1076.
- Bergmeyer HU, Bernt E (1974): Methods of Enzymatic Analysis, Vol. 2, 2nd ed. New York, Academic Press, pp. 735–763.
- Bhakta T, Mukhejee PK, Mukherjee K, Banerjee S, Subhash CM, Maity TK, Pal M, Saha BP (1999): Evaluation of hepatoprotective activity of Cassia fistula. leaf extract. J Ethnopharmacol 66: 277–282.
- Charya MAS, Reddy SM, Kumar BP, Reddy SR (1979): Laboratory evaluation of some medicinal plant extracts against two pathogenic fungi. New Botany 6: 171–173.
- Chiu D, Lubin B, Shohlet SB (1982): Peroxidative reactions in red cell biology. In: Pryor WA, ed., Free Radicals in Biology. Q3 Vol III. New York, Academic Press, pp. 115–153.
- Comporti M (1985): Lipid peroxidation and cellular damage in toxic liver injury. Lab Invest 53: 599–623.
- Coon MJ, Ding X, Pernecky SJ, Vaz AD (1992): Cytochrome P-450. Progress and predictions. FASEB J 6: 669–673.
- Desai DJ (1984): Vitamin E analysis methods for animal tissues. Meth Enzymol 105: 138–147.
- Dubey GR, Shah RC, Kamat SR, Mahashur AA, Ghormade ST, Jehangir RP, Adrianwala SD, Maskati BT (1985): A study of efficacy and toxicity of short-course chemotherapy with ethambutols rifampicin, pyrazinamide and isoniazid in pulmonary tuberculosis. Lung India 3: 115–122.
- Ellman GL (1959): Tissue sulphydryl groups. Arch Biochem Biophys 82: 70–77.
- Fee JA, Briggs RG (1975): Studies on the reconstitution of bovine erythrocyte superoxide dismutase. V. Preparation and properties of derivatives in which both zinc and copper sites contain copper. Biochim Biophys Acta 400: 439–450.
- Flohe L (1982): Glutathion peroxidase brought into focus. In: Pryor WA ed., Free Radicals in Biology. New York, Academic Press, pp. 223–254.
- Habig WH, Pabst MJ, Jakoby WB (1974): Glutathione-S.-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139.
- Halliwell B (1990): How to characterize a biological antioxidant? Free Radic Res 9: 1–32.
- Halvorsen BL, Holte K, Myhrstad MCW, Barikmo I, Hvattum E, Remberg SF, Wold AB, Haffner K, Baugerod H, Andersen LF, Moskaug J, Jacobs DR, Blomhoff R (2002): A systematic screening of total antioxidants in dietary plants. J Nutr 132: 461–471.
- Hasten B (1983): Flavonoids: A class of natural products of high pharmacological potency. Biochem Pharmaol 32: 1141–1148.
- Hoek JB, Pastorino JG (2002): Ethanol, oxidative stress and cytokine-induced liver cell injury. Alcohol 27: 63–68.
- Hussain Z, Kar P, Hussain SA (2003): Antituberculosis drug induced hepatitis: Risk factors, prevention and management. Indian J Exp Biol 41: 1226–1232.
- Ip SP, Ko KM (1996): The crucial antioxidant action of schisandrin B in protecting against CCl4 hepatotoxicity in mice: A comparative study with butylated hydroxytoulene. Biochem Pharmacol 52: 1687–1693.
- Jafri MA, Aslam M, Kalim J, Singh S (2000): Effect of Punica granatum. (Linn.) (flowers) on blood glucose level in normal and alloxan-induced diabetic rats. J. Ethnopharmacol 70: 309–314.
- Kaplan M, Hayek T, Raz A, Coleman R, Dornfeld L, Vaya J, Aviram M (2001): Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J Nutr 13: 2082–2089.
- Kaplowitz N (2002): Biochemical and cellular mechanisms of toxic liver injury. Semin Liver Dis 22: 137–144.
- King EJ, Armstrong AJ (1934): Convenient method for determining serum and bile phosphorous activity. Can Med Assoc J 31: 376–381.
- King J (1965): The dehydrogenase of oxido-reductase-lactate dehydrogenase. In: Van D, ed., Practical Clinical Enzymology. London, Nostrand, pp. 83–93.
- Lee WM (1995): Drug-induced hepatotoxicity. New Engl J Med 333: 1118–1121.
- Mahle C, Dasgupta A (1997): Decreased total antioxidant capacity elevated lipid hydroperoxide concentrations in sera of epileptic patients receiving phenytoin. Life Sci 61: 437–443.
- Misra HP, Fridovich I (1972): The role of superoxide anion in the auto oxidation of epinephrine and a simple assay of superoxide dismutase. J Biol Chem 247: 3170–3175.
- Mitchell JR, Thorgeirsson UP, Black M, Timbrel JA, Snodgrass WR, Potter WZ, Jollow DJ (1975): Increased incidence of isoniazid hepatitis in rapid acetylator: Possible relation to hydrazine metabolites. Clin Pharmacol Ther 18: 70–79.
- Noda Y, Kaneyuki T, Mori A, Packer L (2002): Antioxidant activities of pomegranate fruit extract and it's anthocyanidins: Delphinidin, cyanidin and pelargonidin. J Agric Food Chem 50: 166–171.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Omaye ST, Turnbull TO, Sauberlich HE (1979): Selected methods for the determination of ascorbic acid in cells, tissue and fluids. Meth Enzymol 62: 3–11.
- Prabakan M, Anandan R, Devaki T (2000): Protective effect of Hemidesmus indicus against rifampicin and isoniazid induced hepatotoxicity in rats. Fitoterapia 71: 55–59.
- Prashanth D, Asha MK, Amit A (2001): Antibacterial activity of Punica granatum.. Fitoterapia 72: 171–173.
- Ravikumar V, Shivashangari S, Devaki T (2005): Effect of Tridax procumbens. on liver antioxidant defense system during lipopolysaccaride induced hepatitis in D-galactosamine sensitized rats. Mol Cell Biochem 269: 131–136.
- Ross GR, Selvasubramanian S, Jayasundar S (2001): Immunomodulatory activity of Punica granatum. in rabbits – a preliminary study. J Ethnopharmacol 78: 85–87.
- Rotruck JT, Pope AL, Ganther HE, Hafeman DG, Hoekstra G (1973): Selenium biochemical role as a component of glutathione peroxidase. Science 179: 588–590.
- Schubert SY, Lansky EP, Neeman I (1999): Antioxidant and eicosanoid enzyme inhibition properties of pomegranate seed oil and fermented juice flavonoids. J Ethnopharmacol 60: 11–17.
- Schwab GE, Raucy JL, Johnson EF (1988): Modulation of rabbit and human hepatic cytochrome P-450 catalyzed steroid hydroxylation by α-napthoflavone. Mol Pharmacol 33: 493–499.
- Shingadia D, Novelli V (2003): Diagnosis and treatment of tuberculosis in children. Lancet 3: 624–663.
- Singh SP, Janek AJ, Srivastawa SK, Awasthi S, Awasthi YC, Xia SJ, Zimniak P (2002): Membrane association of glutathione-S.-transferase mGSTA 4-4, an enzyme that metabolizes lipid-peroxidation products. J Biol Chem 277: 4232–4239.
- Singhal KC (1983): Anthelmintic activity of Punica granatum. and Artemesia siversiana. against experimental infection in mice. Ind J Pharmacol 15: 119–122.
- Stork CM, Hoffman RS (1996): In: Rom WN, Garay SM, eds., Tuberculosis. New York, Little, Brown, pp. 829–830.
- Takahara S, Hamilton BM, Nell JV, Ogura Y, Nishmura ET (1960): Hypocatalasemia, a new genetic carrier states. J Clin Invest 29: 610–619.
- Watkins PB (1990): Role of cytochrome P-450 in drug metabolism and hepatotoxicity. Semin Liver Dis 10: 235–250.
- Yamamoto T, Suou T, Hiryama C (1986): Elevated serum aminotransferase induced by isoniazid in relation to isoniazid acetylator phenotype. Hepatology 6: 295–298.
