Abstract
An aqueous preparation from Acacia arabica. var. indica. Benth (Mimosaceae) (locally known as “babul”) leaves (BExt) was assessed for its in vitro. antiviral activity using peste des petits ruminants. virus (PPRV) as a test model in the Vero cell system. Cytopathic effect (CPE) inhibition, virus titration, cell ELISA, sandwich-ELISA (s-ELISA), and PCR assays were used to determine the antiviral effects (at maximum noncytotoxic concentrations 150 and 200 μg/mL) against PPRV, and in vitro. cytotoxicity assays established the relative safety concentration of the BExt for the cells. BExt inhibited viral infectivity drastically in terms of decreased virus titer and antigen load in a dose-dependent manner either when added to cell monolayers postinfection or when preincubated with virus before adsorption on the cells. Inhibition of cell-free and cell-associated PPRV during replication in presence of BExt in Vero cells, using a multistep growth curve experiment, were assessed by s-ELISA. BExt (200 μg/mL) completely inhibited PPRV replication in Vero cells that were infected with PPRV at 0.01 multiplicity of infection. Incubation of PPRV with BExt (150 and 200 μg/mL) followed by infection had a virucidal effect on subsequent progeny virus yield by a 3 log10 TCID50 reduction. This indicates that active principle(s) of BExt either inactivated the virus or inhibited the viral release. Real-time PCR data based on nucleoprotein gene showed 196.7-and 770.6-fold reduction in the viral load in the presence of BExt concentrations of 150 and 200 μag/mL, respectively, indicating the efficacy of BExt in inhibiting PPRV multiplication. These data suggest that extracts of A. arabica. could be a potential natural antiviral agent for management of PPR disease and also a possible addition in the traditional phyto-antiviral repertoire for viral disease control.
Introduction
Peste des petits ruminants.(PPR) is an acute and highly contagious, economically important viral disease of small ruminants, especially goats and sheep, and is caused by PPR virus (PPRV; genus Morbillivirus, family Paramyx-oviridae) (Van Regenmortel et al., Citation2000). Morbidity and mortality rates in small ruminants vary but can be as high as 100% and 90%, respectively (Abu-Elzein et al., Citation1990). PPR is being controlled by using the most effective 55 homologous tissue culture vaccines (Diallo et al., Citation1989; Sreenivasa et al., Citation2000; Sarkar et al., Citation2003) as a prophylactic measure. Though, specific antiviral treatments for herpesvirus infections are available (Elion et al., Citation1997), no such therapy is available for other viral infections including PPR. In the past few decades, as an alternative to conventional chemical agents, a large number of phytochemicals have been assessed for their potential antiviral activity (Kalvatchev et al., Citation1997; Yamasaki et al., Citation1998; Abad et al., Citation1999a, Citationb, Citation2000). Acacia arabica. var. indica. Benth (vernacular names: babul tree/wattle bark, amrad gum, thorny mimosa of India) plant belongs to Mimosaceae and is well-known for its medicinal value since historic times. The A. arabica. extract has been reported to have antimicrobial (Almas, Citation2001), antifertility (Azad Chowdhury et al., Citation1984), and hypoglycemic (Singh et al., Citation1975; Wadood et al., Citation1989) activities. In search of safe and effective natural antiviral agents, in the current study an aqueous extract from A. arabica. leaves (BExt) was tested for its antiviral activity against a model morbillivirus—the PPRV.
Materials and Methods
Plants and preparation of extract
The leaves of babul plant were collected, washed, and dried at room temperature under shade. After overnight incubation in a hot air oven (70°C), the dried leaves were pulverized into a fine powder using an electric grinder/ Waring blender. The liquid extract was dried in a rotatory vacuum evaporator at 45°C to yield crystalline powder. The powdery crude extract was dissolved in Eagle's minimum essential medium (EMEM) to prepare a concentration of 20 mg/mL, stirred for 1 h and, the suspension was centrifuged at 500 × G for 10 min to remove any debris. The supernatant was subsequently filtered through an 0.22 μm membrane filter and stored at −20°C in aliquots for further use.
Phytochemical analysis
Prepared extract (BExt) was tested for the presence of alkaloids, anthraquinone, flavonoids, saponins, tannins, reducing sugars, and proteins as per standard methods (Peach & Tracey, Citation1956; Das et al., Citation1964; Harborne, 1973).
Cells and viruses
Vero cells (CCL-81) between the 140th and 150th pas-sages were propagated in EMEM supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Maintenance medium containing 2% FBS was used for maintenance of cells. The PPR vaccine virus (attenuated Sungri 96 isolate, 60th passage) adapted to Vero cells (Sreenivasa et al., Citation2000) was used as virus source. The Vero cell monolayer was infected with PPRV at 0.01 multiplicity of infection (m.o.i). and allowed to adsorb at 37°C for 1 h. Then the monolayer was fed with maintenance medium and incubated for 4–6 days until > 80% CPE was observed with a change of medium every alternative day. The virus was harvested when infected Vero cells showed PPRV-specific cytopathic effects (CPE) (syncytia, ballooning, and rounding of cells). After harvesting, freezing, and thawing was done three-times, the virus was titrated in Vero cell monolayer. The titer was calculated (Reed & Muench, Citation1938) and expressed as log tissue culture infective dose 50% (TCID50). The titrated virus was stored in aliquots at −20°C until further use.
Cytotoxicity assay: Determination of the maximum nontoxic concentration (MNTC)
The cytotoxicity of crude BExt was determined following the method of Walker et al. (Citation1971). Various concentrations (50, 75, 100, 150, 200, 400, 500, 600, 700, 1000μ ag/mL) of BExt were prepared in maintenance medium and added to the 24 h confluent Vero cell mono layers in 12-well tissue culture plates and incubated in an atmosphere of 5% CO2 at 37°C for 4 days. Each concentration was tested in triplicate along with controls. Cells were examined daily for morphologic changes (rounding, degeneration) if any. After 96 h, cell morphology was compared between treated and untreated cultures (control). The highest concentration of the extract that showed no cellular morphologic changes was considered as the MNTC. Further, the medium was removed and the cells were trypsinized and counted by the trypan blue dye exclusion staining method to assess the viability of cells. The concentration of the extract at which 50% cytotoxicity (i.e., 50% viability of cells) was observed, was recorded as the CyC50 value. The concentration of extract at which cell viability was above 80% was further used in the study for assessment of antiviral properties against PPRV in a dose-dependent manner.
Antiviral screening assay (cytopathic effect inhibitory assay)
Assessment of in vitro. antiviral activity of BExt was initially performed in 96-well microtiter plates in triplicate. Vero cells were seeded at 5 × 104 cells/well. After 24 h, the growth medium was removed and replaced with serial dilutions of BExt in maintenance medium. The pre-titrated vaccine virus (105.5 TCID50/mL) at different dilutions (10−1 to 10−8) in 100 L volume was used along with various nontoxic concentrations (50, 75, 100, 150, 200, and 400 μg/mL) of extracts. To ensure that the effect monitored was that of the extract alone, a set of controls like virus, extract (at different dilutions), and appropriate cell controls were included in the test. The plates were incubated at 37°C in a humidified CO2 incubator, and maintenance media was changed at every 48 h along with extract. The cells were observed for CPE regularly under microscope. After visual observation of PPRV-specific CPE on the sixth day, the supernatant of each well was discarded gently and cells were fixed with chilled acetone: PBS (80:20) for 10 min (Samuel et al., Citation2000). To confirm the presence of CPE of PPRV in Vero cells (with or with out BExt), a cell-ELISA was also performed (Singh, Citation2002).
Briefly, after fixation, microtiter plates were air-dried and blocked (200 μL/well) with blocking buffer (PBS with 1% gelatin, 0.5% fetal calf serum, and 0.1% Tween-20) and incubated for 1 h. In each step, the plates were incubated at 37°C for 1 h under constant shaking. After incubation, the wells were washed three-times with washing buffer (0.002 M PBS+ 0.05% Tween-20). After incubation and washing, PPRV-specific anti-nucleoprotein (N) monoclonal antibody (MAb) at 1:20 dilution (100 μL/well) was added, and the plates were incubated. An anti-mouse horseradish peroxidase (HRPO) conjugate (Dako Cytomation, Glostrup, Denmark) diluted 1:1000 in blocking buffer without gelatin was added (100 μL/well) and the plates were incubated at 37°C for 1 h. The substrate solution [0.4 mg/mL of o.-phenylene-diamine (OPD) containing 4 μL of 3% H2O2] was added at 100 μL/well. The color was allowed to develop for 15 min, after which the reaction was stopped with 1 M H2SO4. Absorbance (A492) was read in an ELISA reader (Labsystems Multiskan Plus, Helsinki, Finland) and cutoff value with two-times the absorbance compared with uninfected cells was set for declaring a well positive.
Further evaluation of antiviral activity (inhibition of viral CPE) of BExt was done in a similar manner using preformed Vero cell monolayer in 25-cm2 flasks, which were infected with 0.01 m.o.i. virus and treated with different nontoxic concentrations of extract, and the mixture was incubated in a 5% CO2 atmosphere at 37°C. After 6 days of incubation, the virus was harvested. The virus titers (TCID50) of the harvests were estimated for each concentration of BExt used along with appropriate controls. Each experiment was run in triplicate together with infected-untreated and uninfected-treated controls.
The antiviral activity was expressed as percentage of inhibition (PI) (Nishimura et al., Citation1977) using antilogarithm values of TCID50, as: PI = [1 − (T antilogarithm/C antilogarithm)] × 100. The extract concentration was considered active when it showed PI higher than 80% at MNTC. The effective concentration capable of reducing CPE by 50% (EC50) was calculated by estimating the virus titer in comparison with virus controls. The selectivity index (SI) was calculated as: SI = 50% cytotoxic concentration (CyC50)/EC50. Virus titer was determined by microplate assay as per the standard methods described by Mariner et al. (Citation1990).
Sandwich-ELISA
After 5–6 days of infection, the harvested samples were tested in s-ELISA (Singh et al., Citation2004) to assess the viral antigen load. The assay was carried out using an anti-RPV rabbit capture antibody at 1:4000 dilution (100 μL) in PBS in 96-well flat-bottom polystyrene microtiter plates (Maxisorp; Nalgene Nunc Int., Raskilde, Denmark). After incubation, the wells were washed three-times with washing buffer (0.002 M PBS+ 0.05% Tween-20). Then, 50 μ L of a blocking buffer (PBS+ 0.1% Tween-20 + 0.2% PPR antibody negative serum) was added to each well. Harvested samples (50 μL/well) were added in duplicate along with positive (PPRV) and negative (uninfected Vero cell supernatant) controls. Subsequent steps were similar as for cell ELISA.
RNA extraction and one-step RT-PCR
Total RNA was extracted from PPRV-infected cell cul-230 ture harvests [> 80% CPE after 5 to 6 days postinfection (dpi)] treated with various concentrations of BExt or without BExt treatment (as control) using Tri-Reagent LS (Sigma-Aldrich, USA) with necessary modifications (Chomczynski & Sachhi, Citation1987). Extracted RNA was subjected to RT-PCR using one-step RT-PCR kit (Qiagen Inc, Valencia, CA, USA) as described earlier (Balamurugan et al., Citation2006) using 20 pmol PPRV-specific primers with prescribed PCR conditions. The sensitivity of this assay was 100 femtogram viral RNA for detection of PPRV. The PPRV N-gene primers (NP3a and NP4) (Couacy-Hymann et al., Citation2002) and F-gene primers (F1 and F2) (Forsyth & Barrett, Citation1995)] were used in the study. The NP3a primer (TCTCGGAAATCGCCTCGCAGGCTG) was a modified sequence of published NP3 primer, which was based on Asian lineage-4 PPRV sequences (AY560591). RT-PCR products were analyzed in 1.5% agarose gels along with standard MW DNA marker.
Real-time PCR
To assess the quantity of viral genome template in the harvests, real-time PCR using N-gene-specific primers (NP3a and NP4) was also performed. Reverse transcription (RT) was done using MMLV reverse transcriptase (Promega Corporation, Madison, WI, USA) and random hexamers (100 pmol) at 37°C for 1 h. Subsequent real-time PCR was carried out in 25 μ L volume using 10 pmol concentration of primers and QuantiTect SYBR Green PCR Master Mix containing HotStarTaq DNA polymerase (Qiagen Inc) in Mx 3000 p machine (Stratagene Inc, LaJolla, CA, USA). PCR was carried out for 30 cycles: denaturation at 94°C for 30s, primer annealing at 55°C for 60s, and extension at 72°C for 60s with an initial activation of enzyme at 95°C for 15 min. Mean cycle threshold (ACt) values of duplicate samples were used for analysis. Melting curves of the PCR products were deduced for calculation of dissociation curves. The entire analysis was done using the inbuilt software program of the M × 3000 p machine.
Virucidal assay
The effects of BExt on inactivation of PPRV infectivity were evaluated by direct contact assay. Briefly, PPRV (2 × 104 TCID50) in 1 mL of maintenance medium was mixed with either 150 or 200 μg/mL extract and incubated for 2 h at 25°C along with virus control without extract. Upon dilution (mixed solution was diluted 10-fold), the surviving infectious virus or residual virus infectivity was determined using the titration method as described previously and was compared with the mock-pretreated control.
Multistep growth curve
To examine the antiviral activity of the extract at various stages of viral replication, a growth curve experiment was also carried out with high nontoxic concentration of BExt (at 150 and 200 μ/mL) using standard protocols. In order to study the growth curve of the PPRV, confluent monolayers of Vero cells in 6-well plates were infected with PPRV at 0.01 m.o.i. in 2 mL maintenance medium containing BExt either by adsorption or virucidal methods along with virus control without extract. Culture supernatant, cell, and total extracts from infected wells were harvested at an interval of 24 h up to 6 days. The infected cells were dislodged by pipetting in 2 mL of fresh medium, and the resulting cell extract was harvested. All the samples (supernatant, cell, and total extracts) harvested at different intervals postinfec-tion were subjected to freezing and thawing three-times, and the viral antigen load was analyzed by s-ELISA.
Results and Discussion
Natural products from many traditional plants have been documented as a relevant source of antiviral drugs (Abad et al., Citation2000; Donia & Hamann, Citation2003). For example, antiviral activities of many phytochemicals have been described for vesicular stomatitis virus (VSV) (Chiba et al., Citation1992; Eo et al., Citation1999, Citation2001). Similarly, gall extract from Guiera senegalensis. J. F. Gmel (Combretaceae) inhibits fowlpox virus-(FPV) (Lamien et al., Citation2005a, Citationb), olive leaf extract inhibits hemorrhagic septicemia rhabdovirus-(VHSV) (Micol et al., Citation2005), and the extract of Dunbaria bella. Prain (Fabaceae) and Euphorbia thymifolia. have been found to be active against herpes viruses (Yang et al., Citation2005; Akanitapichat et al., Citation2006). Babul is one of the popular medicinal plants in India and is widely used as an ethno-veterinary therapeutic agent. With this backdrop, we focused on BExt and assessed its potential antiviral activity in vitro. against PPRV.
Before assessing antiviral activity, cytotoxicity of BExt on Vero cells was studied. After incubating cells for 96 h with concentrations ranging from 50 to 1000 μg/mL, cell viability was determined using the Trypan blue dye exclusion method. On microscopic observation of cell morphology, rounding of cells was observed in 75% of Vero cells at 400 μg/mL concentration of BExt, whereas only 25% of cells showed rounding at 150 and 200 μg/mL concentration. The concentration range in which the extracts did not induce significant toxicity to the host cells varied from 50 to 200 μg/mL of BExt. However, the 50% cytotoxic concentration (CC50 or CyC50) value of the aqueous BExt for Vero cells was 500 μg/mL. However, the viability of Vero cells was further reduced after using the extract at concentration above 500 μg/mL concentrations (). To determine a nontoxic concentration of BExt, Vero cells, maintained at MNTC 150 and 200 μg/mL of BExt for 5 to 6 days with change of media containing the same concentration of extract at every 48 h, were subjected to subculture with normal medium without extract. The viable state of Vero cells even after the sixth day of maintenance with BExt proved that the BExt is nontoxic at 150 and 200 μg/mL concentrations. Preliminary phytochemical analysis of BExt revealed that alkaloids, anthraquinone, flavonoids, and reducing sugars were absent, whereas proteins, saponins, and tannins were present. However, ongoing experiments are being performed to identify the antiviral bioactive compounds.
Table 1 Cytotoxicity babul aqueous extract on Vero cells.
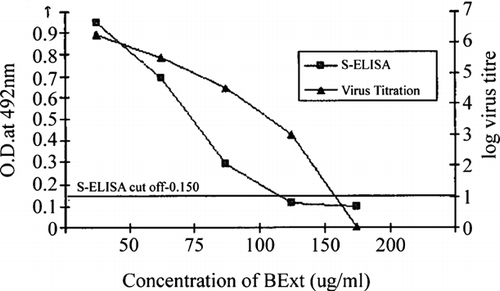
The inhibition of PPRV-induced CPE in Vero cells was observed at 75 μg/mL or higher concentrations with complete inhibition at 200 μg/mL of BExt. More than 80% inhibition was considered to have a significant anti-PPRV activity. The extract at concentrations of 75, 100, and 150 μg/mL had 90%, 99%, and 99.9% inhibition, respectively. The percentages of live virus reduction with 50 and 75 μg/mL of BExt were 43.76% and 90%, respectively. The virus titers were 106,25 TCID50/mL and 105,5 TCID50/mL in the presence of BExt at concentrations of 50 and 75 μg/mL, respectively, as against virus control (106,5 TCID50/mL) without BExt after 6 days postinfection with selectivity indices (SI) of 5 and 6.66 with 50 and 75 μg/mL of BExt, respectively. The reduction in PPR viral antigen in Vero cells by BExt was also confirmed by a Mab-based s-ELISA (Singh et al., Citation2004).
The samples harvested at the sixth day postinfection carrying 100 μg/mL of BExt were found positive in ELISA. In vitro. studies also showed the ability of the extract to inhibit PPRV infectivity titers and to reduce viral antigen load in a dose-dependent manner when added to cell monolayers (). Further, results showed that the BExt inhibits PPRV when the mono-layers were infected either with PPRV preincubated with the extract or when the extract was added after virus adsorption. Decreased sensitivity of the virus to BExt was also noticed when high (0.1) m.o.i. of PPRV was used. The level of inhibition depends on m.o.i. of virus and the duration of the assay. The EC50 values of the extract were 75 and 150 μg/mL when the cells were infected at 0.01 and 0.1 m.o.i., and incubated, respectively, for 6 and 5 days. Similar observation, that is, the antiviral activity of extracts based on reduction of m.o.i. of virus infection, was also reported for fowlpox virus with acetone extract from galls of Guiera senegalensis.(Hu & Hsiung, Citation1989; Rosenwirth et al., Citation1995; Lamien et al., Citation2005a). The EC50 values were considerably less than the CyC50 (high SI), suggesting more in vitro. efficacy and safety. Published literature on natural and synthetic antiviral compounds reveals that the activity against HSV was often dependent on m.o.i. (Akanitapichat et al., Citation2000; del Barrio & Parra, Citation2000; Lowden & Bastow, Citation2003) and/or origin of host cell (Serkedjieva & Ivancheva, Citation1999; del Barrio & Parra, Citation2000). The current results indicated that inhibition of PPRV by BExt was much greater with lower (0.01) m.o.i.
The ELISA negative samples (sixth day postinfection harvested samples treated with BExt above at 150 μg/mL concentration) were tested in one-step RT-PCR assay based on F and N genes of PPRV for the detection of viral genomic template. The F-gene-based PCR resulted in specific amplification of PPRV F-gene fragments of 372 bp at a concentration of 150 μg/mL, but no amplification signal was seen at 200 μg/mL concentration of BExt (). Moreover, Cattaneo et al. (Citation1987) and Ghosh et al. (Citation1995) have demonstrated steep gradient in the number of copies of each mRNA, from the most abundant 3′-proximal N-gene to the least abundant 5′-proximal L-gene in the cells infected with measles virus and RPV. Therefore, for the detection of the PPRV, a PCR specific to the genes, which are close to the 3′-end of the PPRV genome, would be more appropriate than F-gene. Accordingly, PCR based on PPRV N-gene was also carried out with infected Vero cells in the presence of extract at 150 and 200 μg/mL. This resulted in the specific amplification of PPRV N-gene fragments of 351 bp () with no amplification signal in uninfected Vero cells. The PCR amplification of PPRV N-gene at 150 and 200 μg/mL concentrations and no amplification of PPRV F-gene at 200 μg/mL could be due to much higher number of copies of mRNAs of N-gene than F-gene in the virus-infected cell as reported elsewhere (Cattaneo et al., Citation1987; Ghosh et al., Citation1995).
Figure 2 Agarose gel electrophoresis of RT-PCR products from infected Vero cells with or without BExt using PPRV-specific primers (A) Fl and F2 and (B) NP3a and NP4. (A, B) Lane 1: PPRV grown vero cell without BExt as positive control Lane 2: Uninfected vero cell as negative control. Lane M: 100 bp plus DNA ladder (MBI, Fermentas, USA). (A) Lanes 3, 4 and 5: PPRV grown Vero cell with BExt at concentrations of 100, 150, and 200 μg/mL, respectively. (B) Lanes 3 and 4: PPRV grown Vero cell with BExt at concentrations of 150 and 200 μg/mL, respectively.
Because of positivity of the N-gene-specific products at 200 μg/mL concentration in one-step RT-PCR, the harvested samples were further subjected to real-time PCR to quantify the load of virus template and also to monitor the differential amplification pattern of PPRV N-gene at various concentrations of BExt. The real-time PCR amplification plot indicated that ACt between the virus control and Bext-treated cells was about 7.62 and 9.59 cycles, which was approximately equivalent to 430 196.7-and 770.6-fold reduction in the virus yield in the presence of BExt at 150 μg and 200 μg/mL of concentrations, respectively (). This, in turn, confirms the effectiveness of BExt in the inhibition of PPRV replication. The dissociation curve obtained using PCR products from treated and virus control cells confirmed the presence of specific product of Tm 87.37°C to 87.88°C and the absence of primer-dimers (). However, the healthy control cells/non-template control (NTC) yielded little primer-dimers of Tm 81.76°C/ 78.15°C. Further, on checking final PCR products, 351-bp PPRV N-gene-specific PCR products were also confirmed in treated and virus control cells. The healthy control cells did not yield such amplification (). The control curves fluorescence (dR) or threshold (Ct) was much below the amplification plots of the test samples throughout the run.
Figure 3 Real-time PCR on infected Vero cells with or without BExt using PPRV-specific N-gene primers NP3a and NP4. (A) PCR amplification plot: Green line: PPRV grown without BExt as virus control. Blue line: PPRV grown with 150 μg/mL Bext. Red line: PPRV grown with 200 μg/mL BExt; Gray line: Uninfected Vero cell as negative control. Yellow line: Non-template control. (B) Dissociation curve: Analysis of amplicons derived from melting temperature. (C) Agarose gel electrophoresis of real-time PCR products in 1.5% agarose gel. Lanes 1 and 2: PPRV grown Vero cell with BExt at a concentrations of 200 and 150 μg/mL, respectively, Lane 3: Virus control, Lane 4: Uninfected Vero cell as negative cell control, Lane M: 100 bp plus DNA ladder (MBI, Fermentas).
PPRV-specific CPE was inhibited when PPRV (2 × 103 TCID50/mL) was preincubated at 25°C for 2 h with BExt (150 and 200 μg/mL). When PPRV was preincubated with BExt before adding to the Vero cell mono-layers, the titer of virus control without BExt was 104 TCID50/mL, whereas it was only 10 TCID50/mL with pretreatment. Reduction of titer by 3 log10 TCID50 after incubation of virus with plant extract implies the virucidal activity of the extract on PPRV. The interaction of olive leaf extract (oleuropein) with the surface phospholipid bilayers has previously been reported (Paiva-Martins et al., Citation2003); preincubation of VHSV with leaf extract of olive or oleuropein (LExt or Ole) resulted in 70–75% reduction of cell-to-cell fusion at low pH by viral haemorrhagic septicaemia rhabdovirus (VHSV) in uninfected cells, which could therefore be an effect of the Ole on lipid/protein components present on the VHSV envelope involved in membrane fusion. BExt might exhibit similar effect on viral envelope proteins producing PPRV particles with a reduced membrane attachment or fusion capacity or might induce changes on the PPRV envelope, which could, in turn, interfere with the interaction of glycoprotein to anionic phospholipid domains of membranes and therefore inhibit the early fusion steps. The 470 above assumptions are speculative and need experimental verification.
In the multistep growth experiment, characteristic CPE in virus control well started on the third day post-infection in the form of rounding, ballooning, and syncytia, when infected with 0.01 m.o.i. A CPE to the extent of 80–90% with degeneration of infected Vero cells was noticed between the fifth and sixth days postinfection in virus control compared with Vero cells infected and treated with BExt. On assaying the supernatant, cell, and total extracts collected at regular time intervals for viral load by s-ELISA as described above, declining PPRV load was observed during the growth cycle. Further, detection of PPRV antigen in three harvests using s-ELISA showed less viral antigen both in supernatant and cell harvests of infected Vero cell treated with BExt than virus control (, ). The quantity of cell-free virus gradually increased after the third day postinfection dpi and was maximum on the fifth to sixth days postinfection. Cell-associated virus could be detected from the third day postinfection onwards in the infected Vero cells without BExt.
Figure 4 Effect of viral antigen load on the anti-PPRV activity of BExt on extracellular, intracellular, and total viruses quantified by s-ELISA (A) Adsorption method (indirect). (B) Virucidal method (direct).
In the yield-reduction assay, BExt inhibited replication of PPRV with an EC50 of 75 μg/mL. Importantly, as revealed by N-gene-based real-time RT-PCR, the anti-PPRV selectivity of babul extract was 150 μg/mL with a reduction of 196.7-fold. The antiviral activity screening assay showed that the anti-PPRV (intracellular and total virus) activity was detected even when the extract was added 1 h after virus adsorption. Additionally, multistep growth experiments demonstrated that 150 μg/mL concentration of BExt significantly inhibited viral release suggesting that one or more active ingredients might be involved in the process. In conclusion, these preliminary results indicate the anti-PPRV action 505 of BExt. Preincubation of PPRV with BExt at 150 and 200 μg/mL reduced viral infectivity by 103 TCID50/mL indicating that the active principle(s) of extract either inactivated the virus or inhibited the viral release.
These results provide evidence for the potential use of babul extract in antiviral therapeutic applications for PPR control. The antiviral activity of the BExt was ascribed to its effects on reducing virus titer or infectivity. The strong and early virucidal action of BExt suggests that further investigation into the active principle(s) is merited. It is further suggested that studies on isolation, purification, and characterization of the active principles of crude BExt would provide a better understanding of its antiviral mechanism, which would be of paramount significance.
Acknowledgments
The authors wish to thank the Director, Indian Veterinary Research Institute, for providing all the facilities and the staff of the National Morbillivirus Referral Laboratory, Division of Virology, for their help.
References
- Abad M J, Bermejo P, Gonzales E, Iglesias I, Irurzun A, Carrasco L. Antiviral activity of Bolivian plant extracts. Gen Pharmacol 1999a; 32: 499–503
- Abad M J, Bermejo P, Sanchez Palomino S, Chiriboga X, Carrasco L. Antiviral activity of some South American medicinal plants. Phytother Res 1999b; 13: 142–146
- Abad M J, Guerra J A, Bermejo P, Irurzun A, Carrasco L. Search for antiviral activity in higher plant extracts. Phytother Res 2000; 14: 604–607
- Abu-Elzein E ME, Hassanien M M, Al-Afaleq A I, Abd-Elhadi M A, Housain F MI. Isolation of Peste-des-petits-ruminants virus from goats in Saudi Arabia. Vet Rec 1990; 127: 309–310
- Akanitapichat P, Lowden C T, Bastow K F. 1,3-Dihydroxyacridone derivatives as inhibitors of Herpes virus replication. Antiviral Res 2000; 45: 123–134
- Akanitapichat P, Wangmaneerat A, Wilairat P, Bastowc K F. Anti-Herpes virus activity of Dunbaria bella. Prain. J. Ethnopharmacol 2006; 105: 64–68
- Almas K. The antimicrobial effects of seven different types of Asian chewing sticks. Odontostomatol Trap 2001; 24: 17–20
- Azad Chowdhury A K, Khaleque R A, Chakder S K. Antifertility activity of a traditional contraceptive pill comprising Acacia catechu, A. arabica and Tragia involucerta. Indian J Med Res 1984; 80: 372–374
- Balamurugan V, Sen A, Saravanan P, Singh R P, Singh R K, Rasool T J, Bandhyopadhyay S K. One-step multiplex RT-PCR assay for the detection of PPR virus in clinical samples. Vet Res Commun 2006; 30: 655–666
- Cattaneo R, Rebmann G, Baczko K, Ter Meulen V, Billeter M A. Altered ratios of measles virus transcripts in diseased human brains. Virology 1987; 160: 523–526
- Chiba K, Takakuwa T, Tada M, Yoshii T. Inhibitory effect of acylphloroglucinol derivatives on the replication of vesicular stomatitis virus. Biosci Biotechnol Biochem 1992; 56: 1769–1772
- Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987; 162: 56–159
- Couacy-Hymann E, Roger F, Hurard C, Guillou J P, Libeau G, Diallo A. Rapid and sensitive detection of Peste-des-petits-ruminants virus by a polymerase chain reaction assay. Virol Methods 2002; 100: 17–25
- Das P K, Nath V, Gopde K D, Sangal A K. Preliminary phytochemical and pharmacological studies on Coculus hirsutus. Linn. Indian J Me Res 1964; 52: 300–306
- del Barrio G, Parra F. Evaluation of the antiviral activity of an aqueous extract from Phyllanthus orbicularis. J Ethnopharmacol 2000; 72: 317–322
- Diallo A, Taylor W P, Lefevre P C, Provost A. Attenuation d'une souche de virus de la peste des petits ruminants: Candidat pour un vaccin homologue vivant (attenuation of a virulent PPR strain: Potential homologous live vaccine). Rev Elev Med Vet Pays Trap 1989; 42: 311–319
- Donia M, Hamann M T. Marine natural products and their potential applications as anti-infective agents. Lancet Infec Dis 2003; 3: 338–348
- Elion G B, Furman J A, de Fyfe P, Miranda L B, Schaeffer H J. Selectivity of action of antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci USA 1997; 74: 5716–5720
- Eo S K, Kim Y S, Lee C K, Han S S. Antiviral activities of various water and methanol soluble substances isolated from Ganoderma lucidum. J Ethnopharmacol 1999; 68: 129–136
- Eo S K, Kim Y S, Oh K W, Lee C K, Lee Y N, Han S S. Mode of antiviral activity of water-soluble components isolated from Elfvingia applanata. on vesicular stomatitis virus. Arch Pharm Res 2001; 24: 74–78
- Forsyth M A, Barrett T. Detection and differentiation of rinderpest and Peste-des-petits-ruminants viruses in diagnostic and experimental samples by polymerase chain reaction using P and F gene-specific primers. Virus Res 1995; 39: 151–163
- Ghosh A, Joshi V P, Shaila M S. Characterization of an in vitro. transcription system from rinderpest virus. Vet Microbiol 1995; 44: 165–173
- Harbone J B. Phytochemical Methods. Chapman and Hall, Castle House Publication Ltd., London 1973; 131–135
- Hu J M, Hsiung G D. Evaluation of new antiviral agents. Part I: In vitro. perspectives. Antiviral Res 1989; 11: 217–232
- Kalvatchev Z, Walder R, Garzaro D. Anti-HIV activity of extracts from Calendula officinalis. flowers. Biomed Pharmacother 1997; 51: 176–180
- Lamien C E, Meda A, Mans J, Romito M, Nacoulma O G, Viljoen G J. Inhibition of fowlpox virus by an aqueous acetone extract from galls of Guiera senegalen-sis. J. F. Gmel (Combretaceae). J Ethnopharmacol 2005a; 96: 249–253
- Lamien C E, Mans J, Meda A, Couacy-Hymann E, Romito M, Ouedraogo A G, Nacoulma O G, Viljoen G J. In ovo inhibition of fowlpox virus replication by a gall extract from Guiera senegalensis. Avian Pathol 2005b; 34: 127–132
- Lowden C T, Bastow K F. Cell culture replication of Herpes simplex virus and, or human cytomegalovirus is inhibited by 3,7-dialkoxylated 1-hydroxyacridone derivatives. Antiviral Res 2003; 59: 143–154
- Mariner J C, House J A, Soiled A E, Stem C, van den Ende M, Mebus C A. Comparison of the effect of various chemical stabilizers and lyophilization cycles on the thermostability of a Vero cell-adapted rinderpest vaccine. Vet Microbiol 1990; 21: 195–209
- Micol V, Caturla N, Perez-Fons L, Mas Vicente, Perez L, Estepa A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antiviral Res 2005; 66: 129–136
- Nishimura T, Toku K, Fukuyasu H. Antiviral compounds. XII. Antiviral activity of aminohydrazones of alkoxyphenyl substituted carbonyl compounds against influenza virus in eggs and mice. Kitasato Arch Exp Med 1977; 50: 39–46
- Paiva-Martins F, Gordon M H, Gameiro P. Activity and location of olive oil phenolic antioxidants in liposomes. Chem Phys Lipids 2003; 124: 23–36
- Peach K, Tracey M V. Modem Methods of Plant Analysis. Springer-Verlag, Berlin 1956; 479
- Reed L J, Muench H A. Simple method of estimation of fifty percent end points. Am J Hyg 1938; 27: 493–497
- Rosenwirth B, Oren D A, Arnold E, Kis Z L, Eggers H J. SDZ 35–682, a new picornavirus capsid-binding agent with potent antiviral activity. Antiviral Res 1995; 26: 65–82
- Samuel D, Megson B, Ftrang M, Appleton H. A micro-titerplate method for isolation and typing of poliovirus using a blue cell ELISA. Virol Methods 2000; 90: 125–133
- Sarkar J, Sreenivasa B P, Singh R P, Dhar P, Bandyopadhyay S K. Comparative efficacy of various chemical stabilizers on the thermostability of a live-attenuated peste des petits ruminants (PPR) vaccine. Vaccine 2003; 21: 4728–4735
- Serkedjieva J, Ivancheva S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum. L. J Ethnopharmacol 1999; 64: 59–68
- Singh K N, Chandra V, Barthwal K C. Hypoglycaemic activity of Acacia arabica, Acacia benthami. and Acacia modesta. leguminous seed diets in normal young albino rats. Indian J Physiol Pharmacol 1975; 19: 167–168
- Singh R P. Production and characterization of monoclonal antibodies to peste des petits ruminants (PPR) virus. Ph.D. thesis, IVRI, Deemed University, IzatnagarIndia 2002; 35–36
- Singh R P, Sreenivasa B P, Dhar P, Bandyopadhyay S K. A sandwich-ELISA for the diagnosis of Peste des petits ruminants (PPR) infection in small ruminants using anti-nucleocapsid protein monoclonal antibody. Arch Virol 2004; 149: 2155–2170
- Sreenivasa B P, Dhar P, Singh R P, Bandyopadhyay S K. Evaluation of an indigenously developed homologous live attenuated cell culture vaccine against Peste-des-petits-ruminants infection of small ruminants. Proceedings of XX Annual Conference of Indian Association of Veterinary Microbiologists, Immunologists and Specialists in Infectious Diseases (IAVMI), Pantnagar, UttaranchalIndia. Harcourt Science and Technology Company, San Diego 2000; 84
- Van Regenmortel M HV, Fauquet C M, Bishop D HL. Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego 2000, 01.048.1.03
- Wadood A, Wadood N, Shah S A. Effects of Acacia arabica. and Caralluma edulis. on blood glucose levels of normal and alloxan diabetic rabbits. J Pak Med Assoc 1989; 39: 208–212
- Walker W E, Waisbren B A, Martins R R, Batayias G E. A method for determining sensitivities of antiviral drugs in vitro. for possible use as clinical consultation. Am J Clin Pathol 1971; 56: 687–692
- Yamasaki K, Nakano M, Kawahata T, Mori H, Otake T, Ueba N, Oishi I, Inami R, Yamane M, Nakamura M, Murata H, Nakanishi T. Anti-HIV-1 activity of herbs in Labiatae. Biol Pharm Bull 1998; 21: 829–833
- Yang C, Cheng C, Lin T, Chiang C, Lin C C. Euphorbia thymifolia. suppresses Herpes simplex virus-2 infection by directly inactivating virus infectivity. Clin Exp Pharm Physiol 2005; 32: 346–349