Abstract
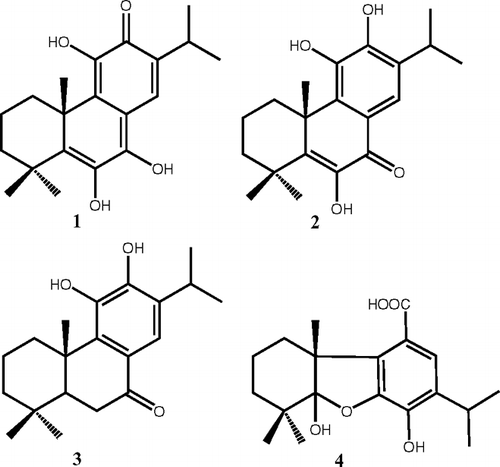
Salvia hypargeia. Fisch. et Mey. (Lamiaceae) root extract, among 16 Salvia. extracts, showed the highest activity against the human ovarian cancer cell line. Bioactivity-guided fractionation of this plant extract has yielded four abietane-type diterpenes [5,6-didehydro-7-hydroxytaxodone (1), 14-deoxycoleon U (= 6-hydroxysalvinolone) (2), demethylcryptojaponol (3), and salvicanaric acid (4)] two triterpenes (lupeol and lupeol-3-acetate), and a fatty acid mixture consisting mainly of palmitic acid (51.6%) and palmitoleic acid (6.4%). Compounds 2 and 3 were found to be active against A2780 human ovarian cancer cell line with IC50 values of 3.9 and 1.2 μ g/mL, respectively, and the fatty acid composition was the most active part of the extract (IC50 = 0.6 μ g/mL).
Introduction
The plant name Salvia. came from Latin word salvare. (“healer”), and the plant has been used against cold and menstrual disorders since ancient times. The genus Salvia. L. (Lamiaceae), with more than 900 species, is distributed all around the world. In Turkey, Salvia. plants are represented by 86 species and 92 taxa (Davis, Citation1982), half of which are endemic.
Salvia. species are a rich source of abietane diterpenes (Rodriguez-Hahn et al., Citation1992; Nagy et al., Citation1999) and possess a number of biological activities including antiseptic, antibacterial (Yang et al., Citation2001), astringent, anti-inflammatory (Baricevic et al., Citation2001), antiviral (Tada et al., Citation1994), cytotoxic (Ryu et al., 1996), cardiovascular, sedative, tranquilizing (Chang & But, Citation1986), spasmolitic, anticonvulsant (Coelho de Souza & Elisabetsky, Citation1998), and carminative. In China, Salvia miltiorrhiza. Bunge has been used in the treatment of coronary heart diseases, particularly angina pectoris and myocardial infarction (Chang et al., Citation1990). Ethnobotanical, phytochemical, and pharmacological studies showed that Salvia. species are considered “cure-all” type plants (Harley et al., Citation1998; Foster & Tyler, Citation2000).
In Turkish folk medicine, especially in rural areas, Salvia. species have been used as stomachic, diuretic, hemostatic, spasmolitic, carminative, and in the treatment of colds and mouth and throat irritations because of its antibacterial and wound healing properties (Baytop, Citation1984). More than 50 Salvia. species have been investigated by our group for their chemical constituents (Ulubelen & Topcu, Citation1998; Topcu & Ulubelen, Citation2007) and when possible for their biological activities (Ulubelen & Topcu, Citation1998; Topcu, Citation2006). We have isolated several antibacterial (Ulubelen et al., Citation2000) and antituberculous active diterpenes (Ulubelen et al., Citation1997) and some cytotoxic abietane and rearranged abietane diterpenes (Ulubelen et al., Citation1999; Topcu et al., Citation2003) and triterpenes (Topcu et al., Citation2004) as well as cardiovascular compounds (Ulubelen, Citation2003) from endemic Turkish Salvia. species.
Since ancient times, some Salvia. species have been used to treat menstrual disorders, and, in Turkey, a few Salvia. species, such as S. virgata. Jacq. have been used in the treatment of vaginitis, uterus cancer, and leukemia (Tuzlaci & Aymaz, Citation2001). Therefore, in this study, a series of extracts of Anatolian Salvia. species were screened for their cytotoxic activity against a human ovarian cancer cell line (A2780) (), and the most active extract was found to be the acetone extract of S. hypargeia. Fisch. et Mey., which is an endemic perennial plant, growing wild in the higher elevations of southern Turkey (Adana). We had previously investigated S. hypargeia. and its closely related species S. montbretii.. The former afforded 14 diterpenoids, and they were tested against a group of cancer cell lines, except ovarian cancer: BC1 (human breast cancer), COL2 (human colon cancer), KB (derived from human nasopharyngeal cancer), KB IV (multidrug-resistant KB), LNCaP (human prostate cancer), P388 (mouse lymphocytic leukemia). Among the isolated 14 diterpenoids, taxodione, 6-hydroxysalvinolone, and saprorthoquinone were found to be active (Ulubelen et al., Citation1999). In the current study, the ovarian cytotoxic activity–guided isolation was carried out, and four active abietane diterpenoids were isolated, and their structures were established by spectral methods, namely based on 1D and 2D NMR experiments.
Table 1 Cytotoxicity of some Salvia. extracts against the A2780 human ovarian cancer cell lineFootnotea..
Materials and Methods
Plant material
All Salvia. species were collected from different regions of Anatolia in their flowering season, especially between June and July (related studies can be found in our previously published studies, some given in the references). The roots of S. hypargeia. Fisch. et Mey. (Labiatae = Lamiaceae) were collected from a highland area of Adana (Tekir-Bürücek) in southern Turkey at an altitude 1850 m and identified by Prof. Dr. Kerim Alpinar, and a voucher specimen is deposited in the herbarium of the Faculty of Pharmacy, University of Istanbul (ISTE 68316).
Equipment
Optical rotations were determined using an Optical Activity Ltd AA-5 polarimeter (Huntington, UK) UV with a Shimadzu 1601 spectrometer (Kyoto, Japan), IR with a Perkin-Elmer model 983, and 1H and 13C NMR on Varian 400 (USA) and Jeol Eclipse 500 spectrometers (Tokyo, Japan). Mass spectra were obtained on a VG-Zabspec (Micromass-VG Analytical, Manchester, UK) instrument. HPLC was performed on a Shimadzu LC-10AT instrument (Japan).
Extraction and isolation
In this study, fifteen Salvia. species, growing in Turkey, were exhausted by maceration in ethanol to study their ovarian cytotoxic activity while a few in other solvents. We used the roots of the plants (S. amplexicaulis. Lam., S. aucheri. Benth., S. bracteata. Banks & Sol., S. candidissima. Vahl, S. heldreichiana. Boiss. ex Benth., S. hypargeia. Fisch. et Mey., S. napifolia. Jacq., S. tomentosa. Mill.) or the aerial parts (S. cassia. G. Samuelsson ex Rech. f., S. eriophora. Boiss. & Kotschy ex Boiss., S. pilifera. Montb. & Auch., and S. recognita. Fisch. & Mey.) for the extraction. However, the whole plant extracts were prepared from S. staminea. Montb. & Auch. ex Benth., S. triloba. L. (S. fruticosa. Mill.) and S. syriaca. L. ().
For the most active plant, S. hypargeia., the air-dried and powdered roots (1 kg) were extracted with acetone in a Soxhlet apparatus. The solvent was removed in vacuo.. The crude acetone extract (58 g) was fractionated on a silica gel (70–200 mesh) column (5 × 75 cm) eluting with n.-hexane (10 × 200 mL) followed by a gradient CH2Cl2 with 5% increments reaching 100%, and followed by a gradient EtOH. The eluted fractions were checked by TLC, and similar fractions were combined to give six fractions. The first five of these fractions have been previously studied (Ulubelen et al., Citation1999), so the final polar fraction was purified in a bioactivity-guided way to afford the four diterpenes 1–4 (), the two triterpenes 5 and 6, and a mixture of fatty acids. Compound 1 was purified by preparative TLC (Si-gel F254 plates) using a CHCl3:MeOH (95:05) solvent system, and a CHCl3:MeOH (90:10) solvent mixture was used for compounds 2 and 3. Compound 4 was purified by HPLC on a reversed-phase C-18 column using the solvent system MeOH:H2O (7:3). All six compounds were identified by comparision of their 1H and 13C NMR data with literature data (Kupchan et al., Citation1968; Hueso-Rodriguez et al., Citation1983; Gonzalez et al., Citation1987; Luis & Grillo, Citation1993; Misra et al., Citation1993; Marques et al., Citation2002). The compounds were identified as 5,6-didehydro-7-hydroxytaxodone (1) (20 mg) [α]D = 0° (MeOH, c. 0.1), 14-deoxycoleon U (6-hydroxysalvinolone) (2) (50 mg) [α]D = 42° (CHCl3, c. 0.1), demethylcryptojaponol (3) (60 mg) [α]D = +31.2° (CHCl3 c. 0.6), salvicanaric acid (4) (13 mg) (acetate methyl ester of 4) [α]D = 72.2°, (CHCl3, c. 0.072), lupeol (34 mg) [α]D = +28° (CHCl3, c. 0.3), and lupeol acetate (45 mg) [α]D = +45° (CHCl3, c. 0.5). GC-MS analysis of the fatty acid mixture indicated the presence of 51.6% hexadecanoic acid (palmitic acid) and 6.4% 9-hexadecenoic acid (palmitoleic acid); the remaining material was composed of more than 20 unidentified fatty acids.
Cytotoxicity assay
Cytotoxicity was determined against A2780 human ovarian cancer cells using a microtiter plate assay. The plates were seeded with cells, and the extracts or compounds (dissolved in 1:1 DMSO:H2O) were added to the cells at specific concentrations. The plates were incubated at 37°C and 5% CO2 for 48 h. Then Alamar blue (Biosource International, Camarillo, CA, USA) was added to the cells, and the plates were incubated for 3 h. During this time, the Alamar blue is taken up by the live cells and reduced. The reduced form of Alamar blue is stable and fluorescent. The fluorescence of each well in the plate was measured. The fluorescence is directly proportional to the percent inhibition of the growth of the cells. The IC50 value was determined by plotting the data on a dose-response curve of percent inhibition versus concentration. The IC50 value is defined as the concentration of sample necessary to produce 50% inhibition of the growth of the cells. Lower IC50 indicate greater activity of values compounds (Louie et al., Citation1985; Guza, Citation2004).
Results and Discussion
In this study, a number of crude extracts of Turkish Salvia. species including S. hypargeia. () have been screened in a dose-dependent manner against the A2780 human ovarian cell line, and S. hypargeia. extract was found to be the most active one among them. Isolation of cytotoxic constituents from this extract yielded four abietane diterpenoids [5,6-didehydro-7-hydroxytaxodone (1) (Luis & Grillo, Citation1993), 14-deoxycoleon U (= 6-hydroxysalvinolone) (2) (Hueso-Rodriguez et al., Citation1983; Marques et al., Citation2002), demethylcryptojaponol (3) (Hueso-Rodriguez et al., Citation1983), and salvicanaric acid (4) (Gonzalez et al., Citation1987) ()], and two triterpenoids (lupeol and lupeol-3-acetate) (Misra et al., Citation1993), as well as a fatty acid mixture. The activity of the diterpenoids against ovarian cancer cell lines was determined, and the results are given in . The data of indicate that demethylcryptojaponol (3) and 14-deoxycoleon U (2) are highly active, and compounds 1 and 4 are not as active as 2 and 3. Interestingly, the most active component was found to be the fatty acid mixture. Its high cytotoxic activity can be attributed to the presence of its main constituents palmitic and palmitoleic acid, which were found in high percentages in this mixture. In a recent study on melanoma cells, palmitic acid was the most toxic against B16F10 murine melanoma cells, including cell death by apoptosis and necrosis. In fact, the human melanoma cell lines were more resistant to the toxic effect of fatty acids. In SK-Mel 23 cells, cytotoxicity was detected after 48-h treatment with arachidonic, linoleic, palmitic, and palmitoleic acids at 200 μ M, and linoleic acid was found to be the most toxic fatty acid, whereas in SK-Mel 28 human cells, only palmitic acid caused a significant decrease of the number of viable cells inducing DNA fragmentation after 24-h and 48-h treatments at the same concentration (Andrade et al., Citation2005).
Table 2 Cytotoxicity of the isolated abietane diterpenoids and fatty acid mixture against the A2780 cell line.Footnotea.
Compound 4 (salvicanaric acid) is a rearranged abietane diterpenoid, and its formation through demethylcryptojaponol has been postulated previously (Gonzalez et al., Citation1987). It was also isolated from a few Salvia. species including S. montbretii. (Topcu & Ulubelen, Citation1996) and S. munzii Epl.. (Luis et al., Citation1993).
The chemical constituent profiles of S. hypargeia. and S. montbretii. were found to be very similar. 14-Deoxycoleon U (6-hydroxysalvinolone), demethylcryptojaponol, salvicanaric acid, taxodione, ferruginol, microstegiol, and saprorthoquinone were isolated from both species (Topcu & Ulubelen, Citation1996; Ulubelen et al., Citation1999), which verified both plants are close congeners, as mentioned in Flora of Turkey. (Davis, Citation1982). Since the 1960s, taxodione has been known as a potential anticancer agent, which was first presented by Kupchan et al. (Citation1968). 14-Deoxycoleon U (2) and its 14-hydroxylated derivative “coleon U” were also found to be highly active against several cancer cell lines (Marques et al., Citation2002; Cerquira et al., 2004) by other researchers, but these diterpenoids have not been previously tested against ovarian cell lines. Furthermore, demethylcryptojaponol and taxodione showed potent antimicrobial activity with MIC values of 4–10 μ g/mL against methicillin-resistant Staphylococcus aureus. (Yang et al., Citation2001). In a recent study, 14-deoxycoleon U and demethylcryptojaponol were investigated for their antifeedant activity. The former showed strong antifeedant activity against Leptinotarsa decemlineata., whereas the latter was toxic to this insect, but they had no antifeedant or negative effects on Spodoptera littoralis. (Boisd.) (Fraga et al., Citation2005).
On the other hand, in our previous study, most abietane diterpenoids isolated from S. hypargeia. (Ulubelen et al., Citation1999) have been tested against a series of cell lines, except for ovarian cells, and they were found more or less active, and the highest activity was obtained by taxodione against most tested cell lines. However, in the current study, taxodione did not show high activity against the A2780 ovarian cell line. In contrast, demethylcryptojaponol (3) and 14-deoxycoleon U (2) were found to be highly active ovarian cytotoxic compounds with IC50 values of 1.2 and 3.9 μ g/mL, respectively.
Acknowledgments
The authors thank TUBITAK and the National Science Foundation for financial support for this study through the project TBAG U53–INT0002071. One of us (A.U.) thanks the Turkish Academy of Sciences (TUBA) for partial support of this study. We thank Dr. Afgan Farooq and Mr. Mohammed Radwan (VPISU) for their assistance in running NMR spectra.
Notes
*The structure of 6-hydroxysalvinolone was incorrectly drawn without a keto group at C-7 in the paper.
References
- Andrade L N, de Lima T M, Curi R, Castrucci A M. Toxicity of fatty acids on murine and human melanoma cell lines. Toxicology in Vitro 2005; 19: 553–630
- Baricevic D, Sosa S, Della Loggia R, Tubaro A, Simonovska B, Krasna A, Zupancic A. Topical anti-inflammatory activity of Salvia officinalis. L. leaves: The relevance of ursolic acid. J Ethnopharmacol 2001; 75: 125–132
- Baytop T. Therapy with Medicinal Plants of Turkey (Past and Present). Istanbul University Press, Istanbul 1984; 156–158
- Chang H M, But P. Pharmacology and Applications of Chinese Materia Medica, Vol. I. World Scientific Publishing Co., Singapore 1986; 255–268
- Chang H M, Cheng K P, Choang T F, Chow H F, Chui K Y, Hon P M, Tan F WL, Yang Y, Zhong Z P, Lee C M, Sham H L, Chan C F, Cui Y X, Wong H NC. Structure elucidation and total synthesis of new tanshinones isolated from S. miltiorrhiza. Bunge (Danshen). J Org Chem 1990; 55: 3537–3542
- Cerqueira F, Cordeiro-Da-Silva A, Marques C G, Simoes F, Pinto M M, Nascimentoa M S. Effect of abietane diterpenes from Plectranthus grandidentatus. on T-and B lymphocyte proliferation. Bioorg Med Chem 2004; 12: 217–223
- Coelho d e, Souza G P, Elisabetsky E I. Ethnobotany and anticonvulsant properties of Lamiaceae from Rio Grande de Soul (Brasil). Lamiales Newsletter, R Harley, A Payton, T Harvey. Royal Botanic Gardens, Kew 1998; 10
- Davis P H. Flora of Turkey and East Aegean Islands. Vol. 7. Edinburgh University Press, Edinburgh 1982; 461
- Sage. Tyler's Honest Herbal “A sensible guide to the use of the herbs and related remedies.” Fourth ed., S Foster, V E Tyler. The Haworth Press Inc., New York 2000; 327–329; 414–415
- Fraga B M, Diaz C E, Guidano A, Gonzalez-Coloma A. Diterpenes from Salvia broussonetii. transformed roots and their insecticidal activity. J Agr Food Chem 2005; 53: 5200–5206
- Gonzalez A G, Herrera J R, Luis J G, Ravelo A G. Salvicanaric acid, a new diterpene from Salvia canariensis.. J Nat Prod 1987; 50: 341–348
- Guza R C. Isolation of Natural Products from Casearia nigrescens. MS Thesis, Department of Chemistry, Virginia Polytechnic Institute and State University, Blacksburg, VA 2004
- Harley R, Paton A, Harvey T. Lamiales Newsletter. Royal Botanic Gardens, Kew 1998
- Hueso-Rodriguez J A, Jimeno M L, Rodriguez B, Savano G, Bruno M. Abietane diterpenoids from the root of Salvia phlomides.. Phytochemistry 1983; 22: 2005–2009
- Kupchan S M, Karim A, Marcks C. Taxodione and taxodone, two novel diterpenoids quinone methide tumor inhibitors. J Am Chem Soc 1968; 90: 5923–5924
- Louie K G, Behrens B C, Kinsella T J, Hamilton T C, Grotzinger K R, McKoy W M, Winker M A, Ozols R F. Radiation survival parameters of antineoplastic drug sensitive and resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Res 1985; 45: 2110–2115
- Luis J G, Grillo T A. New diterpenes from Salvia munzii.: Chemical and biogenetic aspects. Tetrahedron 1993; 49: 6277–6284
- Marques C G, Pedro M, Simones F, Nascimento M S, Pinto M, Rodriguez B. Effect of abietane diterpenes from Plectranthus grandidentatus. on the growth of human cancer cell lines. Planta Med 2002; 68: 839–840
- Misra T N, Singh R S, Ragini S, Pandey H S, Chandan P, Satyendra S. A new triterpenoid from Vernonia cinerea. Planta Med 1993; 59: 458–460
- Nagy G, Gunther G, Mathe I, Blunden G, Yang M H, Crabb T A. Diterpenoids from Salvia glutinosa S. austriaca S. tomentosa S. verticillata. roots. Phytochemistry 1999; 52: 1105–1109
- Rodriguez-Hahn L, Esquivel B, Cardenas J, Ramamoorthy T P. The distribution of diterpenoids in Salvia.. Advances in Labiatae Science, R. M. Harley, T. Reynolds. Royal Botanic Gardens, Kew 1992; 335–347
- Ryu S Y, Lee C O, Choi S U. In vitro. cytotoxic of tanshinones from Salvia miltiorrhiza.. Planta Med 1997; 63: 339–342
- Tada M, Okuna K, Chiba K, Ohnishi E, Yoshii T. Antiviral diterpenes from Salvia officinalis.. Phytochemistry 1994; 35: 539–541
- Topcu G. Bioactive triterpenoids from salvia. species. J Nat Prod 2006; 69: 482–487
- Topcu G, Ulubelen A. Abietane and rearranged abietane diterpenes from Salvia montbretii.. J Nat Prod 1996; 59: 734–737
- Topcu G, Ulubelen A. Structure elucidation of organic compounds from natural sources using 1D-and 2D NMR techniques. J Mol Struct 2007; 834–836: 57–73
- Topcu G, Altiner E N, Gozcu S, Halfon B, Aydogmus Z, Pezzuto J M, Zhou B N, Kingston D GI. Studies on di-and triterpenoids from Salvia staminea. with cytotoxic activity. Planta Med 2003; 69: 464–467
- Topcu G, Turkmen Z, Ulubelen A, Schilling J K, Kingston D GI. Highly hydroxylated triterpenes from Salvia kronenburgii.. J Nat Prod 2004; 67: 118–121
- Tuzlaci E, Aymaz P E. Turkish folk medicinal plants, Part IV: Gonen (Balikesir). Fitoterapia 2001; 72: 323–343
- Ulubelen A. Cardioactive and antibacterial terpenoids from some Salvia species. Phytochemistry 2003; 64: 395–399
- Ulubelen A, Topcu G. Chemical and biological investigations of Salvia. species growing in Turkey. Studies in Natural Products Chemistry, Part F, Vol. 20, Attu-ur-Rahman. Elsevier, Amsterdam 1998; 659–758
- Ulubelen A, Topcu G, Bozok-Johansson C. Norditerpenoids and diterpenoids from Salvia multicaulis. with antituberculous activity. J Nat Prod 1997; 60: 1275–1280
- Ulubelen A, Topcu G, Chai H B, Pezzuto J M. Cytotoxic activity of diterpenoids isolated from Salvia hypargeia.. Pharm Biol 1999; 37: 148–151
- Ulubelen A, Oksuz S, Kolak U, Topcu G, Goren A C, Voelter W. Antibacterial diterpenes from the roots of Salvia eriophora.. Planta Med 2000; 68: 818–821
- Yang Z X, Kitano Y, Chiba K, Shibata N, Kurokawa H, Doi Y, Arakawa Y, Tada M. Synthesis of variously oxidized abietane diterpenes and their antibacterial activities against MRSA and VRE. Bioorgan Med Chem 2001; 9: 347–356