Abstract
Seven flavonol glycosides and four nonflavonoid compounds were isolated from Epimedium koreanum. Nakai (Berberidaceae), and their effects on the proliferation of rat osteoblast-like (ROB) cells were evaluated with a mitochondrial activity assay. Both flavonol glycosides and nonflavonoid compounds strongly inhibited the proliferation of primary ROB cells at most concentrations (2.0 × 10− 5 mol/L ∼ 1.0 × 10−8 mol/L), and a simple structure-function relationship was also found in these compounds. Considering the antiosteoporotic effect of flavonoids in vivo., these results suggest that the flavonoids may enhance the development of osteoblasts through their active metabolites, and the mechanism between proliferation and differentiation of osteoblasts needs to be further studied to fully evaluate the mechanism of E.. koreanum. in the treatment of osteoporosis.
Introduction
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to weakness of the skeleton and increased risk of fracture. The high prevalence of osteoporosis and the morbidity associated with the resulting fractures has led to major public health problems and social and economic costs. The functions of bones include maintaining blood calcium levels, providing mechanical support to soft tissues, serving as levers for muscle action, and so forth. These functions are accomplished by continuous tissue renewal, called remodeling (Harada et al., Citation2003). Bone metabolic diseases develop when there is an imbalance in the remodeling process, which, in turn, depends on the interactions between osteoblasts and osteoclasts. The rationale for prevention and treatment of osteoporosis is directed along two basic approaches, namely agents preventing bone resorption (estrogen, calcitonin, bisphosphonates, calcium, vitamin D, raloxifene) and those stimulating bone formation (fluoride, anabolic steroids). Among them, estrogen replacement therapy (ERT) has been a popular regime for prevention and treatment of postmenopausal osteoporosis. However, recent evidence suggests that ERT is associated with increased risk of development of breast, ovarian, and endometrial cancer (Nelson et al., Citation2002; Davison et al., Citation2003), leading to the newly developed application of selective estrogen receptor modulator including phytoestrogens (Pinkerton et al., Citation1999). Substantial evidence shows that a class of plant-derived substances, so-called phytoestrogens, have estrogenic activities. Phytoestrogens includes the flavonoid family composed of isoflavones and flavonols derivatives. Due to their ability to bind estrogen receptor (ER), these natural compounds could have positive effect against bone loss.
Herbal medicine has been widely used in orthopedic clinical practice for the treatment of fractures and joint diseases. Herba epimedii., which contains several medically active constituents such as flavonoids and phytosteroids, is a “kidney-tonifying” traditional botanical medicine widely used in China, Japan, and Korea. Based on traditional Chinese medicine theory, “kidney” controls bone and supports gonadal functions. Thus, Herba epimedii. has been traditionally used in many Chinese formulas for antiosteoporosis. Modern research also showed that Herba epimedii. can reduce bone loss in ovariectomized rat model (Ji et al., Citation2001; Jiang et al., Citation2002) as well as in an aged rat (Zhang et al., Citation1999) model. It has previously been demonstrated to have effects on preventing bone loss and increasing osteocalcin and E2 levels in a short-term clinical study involving postmenopausal women (Li et al., Citation2000; Jiang et al., Citation2002). Recently, several in vitro. studies indicated the osteoblastic proliferation stimulating activity of the crude extract, total flavonoids, and main flavonol glycosides from Herba epimedii. [Epimedium brevicornum. Maxim (Berberidaceae), E. koreanum. Nakai (Berberidaceae)] toward osteoblast-like UMR106 cells (Meng et al., Citation2005a and Citationb; Xie et al., Citation2005). However, most of these studies were focused on the crude extract or flavonoids. The current study compared the effects of nonflavonoid constituents and flavonol glycosides isolated from E..koreanum. Nakai on the proliferation of primary osteoblasts and investigated the mechanism of E.. koreanum. Nakai in the process of osteoblast development.
Materials and Methods
E.. koreanum. herb collected in June–July 2003 in a valley located in Xinbin, Liaoning Province, was authenticated by Q.-S. Sun, Professor of Pharmacognosy, Shenyang Pharmaceutical University, China. A voucher specimen (no. 19980816-1) has been deposited in the herbarium of the Shenzhen Research Center of Traditional Chinese Medicine & Natural Products.
Newborn NIH mice were obtained from the Laboratory Animal Center of Guangzhou University of Traditional Medicine. Trypsin and fetal bovine serum were purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), benzylpenicillin, Dulbecco's modified Eagle's medium (DMEM, without phenol red), streptomycin, collagen II, and Alizarin red S were from Sigma (St. Louis, MO, USA). Preparative high-performance liquid chromatograph was obtained from Shimadzu (Japan). Silica gel (Qingdao Haiyang Chemical Co., Ltd, China), Sephadex LH-20 (Amersham Biosciences, USA), and octadecylsilane (ODS) (Fuji Silysia, Japan) were used in column chromatography. All other chemicals were of analytical grade.
Extraction and isolation
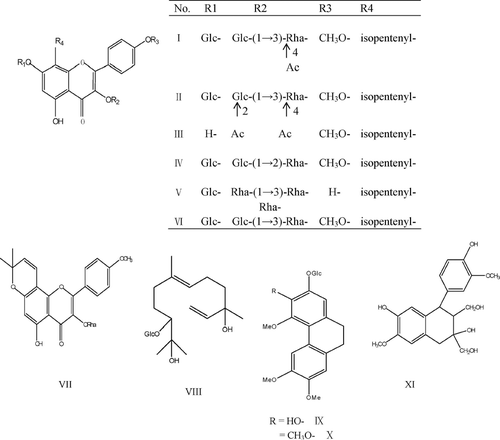
The dried and powdered aerial parts of E. koreanum. (55 kg) were refluxed for 2 h with water (550 L, 2 times). Then, the extract was concentrated and subjected to macroporous adsorptive resins eluted with 0%, 30%, 50%, and 95% ethanol (v/v) which represented yields of 5.5%, 1.7%, 1.2%, and 0.2%, respectively. The flavonoids were concentrated in the 50% fraction identified by coupling HCl-Mg reaction and UV spectra (data not shown). Followed by animal studies, 50% ethanol fraction showed a strong effect on the bone mineral density (BMD) in total and cortical bones (Xie et al., Citation2005). Thus, a part (130 g) of the 50% ethanol fraction was subjected to column chromatography on silica gel. The column was eluted with CHCl3:MeOH in a stepwise manner (99:1 → 1:1) and collected into 16 fractions. Seven flavonol glycosides and four nonflavonoid constituents were isolated from fractions 4 (0.8 g, 97:3), 7 (7.4 g, 90:10), 8 (6 g, 85:15), and 10 (8.7 g, 80:20), respectively, by the combination of Sephadex LH-20 column chromatography, ODS reverse-phase column chromatography, and preparative high-pressure liquid chromatography.
The seven flavonol glycosides were korepimedoside C [I, 35 mg, fraction (fr.) 8), caohuoside E (II, 45 mg, fr. 8), sagittatoside A (III, 15 mg, fr. 10), hexandraside D (IV, 21 mg, fr. 7), epimedoside A (V, 10 mg, fr. 10), hexandraside F (VI, 20 mg, fr. 10), and acuminatin (VII, 8 mg, fr. 4). The four nonflavonoid compounds were icariside C1 (VIII, 15 mg, fr. 7), icariside A5 (IX, 10 mg, fr. 7), epimedoicarisoside A (X, 9 mg, fr. 7), and 1,2,3,4-tetrahydro-3,7-dihydroxy-1-(4-hydroxy-3-methoxyphenyl)-6-methoxy-2,3-Na-phthalenedimethanol (XI, 7 mg, fr. 4). The purity of these compounds was more than 99.0% authenticated by RP-HPLC with Diode Array detector (DAD), and their structures were identified by comparison of their physical properties and IR and NMR spectral data with literature values (Mizuno et al., Citation1988; Sun et al., Citation1998) ().
Preparation of test samples
Samples were dissolved in dimethylsulfoxide (DMSO) at a concentration of 10 mM and diluted in culture medium to the working solution before use. NaF was dissolved in PBS to give concentrations of 10 mM and diluted in culture medium as positive control. To avoid DMSO toxicity, the concentration of the solvent was less than 0.1% (v/v), and with DMSO at the same concentration added to control cultures.
Isolation of primary osteoblasts
Primary osteoblasts were isolated enzymatically from calvariae of newborn NIH mice as described previously (Chen and Fry, Citation1999). Briefly, skulls were dissected, and then endosteum and periosteum were stripped off. The bone was cut into approximately 1–2 mm2 pieces followed by sequential digestion with trypsin (2.5 g/L) for 30 min and collagenase II (1.0 g/L) twice for 1 h each time. The cells were collected and cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μ g/mL streptomycin for 24 h in a humidified atmosphere of 5% CO2 in air at 37°C, then old medium was changed. After reaching 70% confluence, cells were detached by treatment with 0.25% trypsin, replated in 13 cm2 dishes, or 96-well plates (area of each well, 0.28 cm2) at a density of 2.5 × 104 cells/cm2 and grown in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μ g/mL streptomycin. For effects of steroids on growth, medium was charcoal stripped without phenol red. Fresh medium was supplied to cells at 3-day intervals.
Identification of primary osteoblasts
Morphologic characteristics of osteoblasts were identified by histochemical stain: alkaline phosphatase stain and alizarin red S (ARS) stain. A cell alkaline phosphatase (CAKP) staining kit (Kaplow method) was used to detect alkaline phosphatase activity. For the experiment with alizarin red S stain, cells were fixed in 95% ethanol for 30 min at room temperature, washed with PBS, and stained for 20 min with 0.1% alizarin red S, pH 4.2, at room temperature.
Proliferation assay
The MTT assay was used to evaluate mitochondrial activity (Carmichael et al., Citation1987). Briefly, osteoblasts were seeded on a 96-well tissue culture plate and incubated 24 h prior to addition of tested compounds, followed by another 48 h of culture. MTT (20 μ L, 5 mg/mL) were added to the plate 4 h prior to the end of the experiment. Then, the supernatant was removed and DMSO (100 μ L) was added to dissolve purple formazan, and absorbance was measured on a microplate spectrophotometer (Bio-Rad Model 680, USA) at 570 nm with a reference at 655 nm.
Statistical analysis
All data in the text are expressed as the mean ± standard deviation. Statistical significances were analyzed by the Student's t.-test. Statistical significance was set at p < 0.05. Growth inhibition ratios (GIRs) were calculated according to the following equation: GIR% = (A.sample− A.blank)/A.blank× 100, where A. is the average absorbance of four experiments with six replicates.
Results
Identification of primary osteoblasts
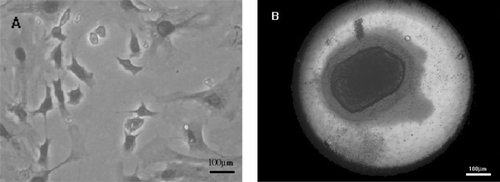
Primary osteoblasts were polymorphological after culturing for 7 d. Cytochemical staining (Keplow method) for alkaline phosphatase in the osteoblasts showed azo dye precipitated in the cytoplasm of osteoblasts. After osteoblasts were subcultured in DMEM containing 10% fetal bovine serum, 10 mM β-glycerophosphate and 50 μ g/mL ascorbic acid, many orange-red mineralized nodules were formed stained by ARS ().
Inhibition of primary osteoblast proliferation
NaF stimulated the proliferation of primary osteoblast to 13.0%, whereas total flavonoids (1.2 × 10− 2 mg/mL to 6.0 × 10− 6 mg/mL) inhibited the proliferation of primary osteoblasts by about 30%. Seven flavonol glycosides (I–VII) and four nonflavonoid constituents (VII–XI) also suppressed the proliferation of primary osteoblasts at most concentrations (2.0 × 10− 5mol/L to 1.0 × 10− 8mol/L) ( and ). For flavonol glycosides (I–VII), the inhibitory ratio was similar under the same concentration except for V and VI, which exhibited stronger inhibitory effects than other test compounds at the same concentration. As to nonflavonoid constituents (VIII–XI), the inhibitory ratio increased as the concentration reduced. It was notable that compound IX at the concentration of 1.0 × 10− 8 mol/L reduced the proliferation of primary osteoblasts to 32.3%. In addition, the inhibitory effects of V and IX were stable and persistent through 2.0 × 10− 5 mol/L to 1.0 × 10− 8 mol/L. Furthermore, there was no significant dose-dependence of the inhibition effect, where the maximum inhibitory effect may arise in the lowest concentration for certain compounds.
Table 1 Effects of flavonol glycosides on osteoblast proliferation.
Table 2 Effects of nonflavonoid constitutents on osteoblast proliferation.
Discussion
Osteoblasts are the skeletal cells responsible for synthesis, deposition, and mineralization of new bone matrix in their differentiated state characterized by the ability of cells to synthesize membrane-associated alkaline phosphatase, bone matrix molecules, a variety of noncollagenous proteins, and so on (Aubin, Citation1998). It has been postulated that bone loss associated with aging is caused by a defect in osteoblast cell lineage (Katzburg et al., Citation1999; Rodriguez et al., Citation1999). The mesenchymal precursor population is either insufficient or has lost the capacity to proliferate and/or differentiate into a sufficient number of functioning osteoblasts.
Several “kidney-tonifying” Chinese herbs have been widely used in China for years to treat bone disease. Recently, in vivo. studies have shown their therapeutic effects on osteoporosis and bone fracture (Hidaka et al., Citation1997; Huang and You, Citation1997; Chen et al., Citation2004; Xie et al., Citation2005). However, results from in vitro. studies were inconsistent with in vivo. studies. The water extract of traditional Chinese herb Drynaria fortunei. (Kunze) J. Smith (Polypodiaceae), for instance, suppressed the proliferation and differentiation of rat osteoblasts (Liu et al., Citation2001). Furthermore, the flavonoid extract from Epimedium sagittatum. (Berberidaceae) has been found to be effective in preventing osteoporosis induced by ovariectomy in rats. However, no appreciable effect was observed when primary osteoblasts were exposed to the flavonoid extract in vitro. (Chen et al., Citation2004; Zhang et al., Citation2008). In addition, serum isolated from rats orally administered with flavonoid extract from E. sagittatum. was able to significantly stimulate the proliferation as well as the differentiation of ROB cells, which indicated that the flavonoids may enhance the development of osteoblasts through their active metabolites (Chen et al., Citation2004).
Recently, several studies revealed that flavonol glycosides simulated the proliferation of UMR106 cells (Meng et al., Citation2005a and b; Xie et al., 2005). In this study, both flavonol glycosides and nonflavonoid constituents were found to inhibit the proliferation of primary osteoblasts. This diserepancy might be the cause of different cell type and culture medium (effects of steroids on growth). In addition, the metabolites generated by flavonol glycosides in vivo. may be the actual active constituents stimulating the proliferation of osteoblasts.
At the same time, a simple structure-function relationship was found in these compounds. For example, comparing the structures among I, II, and VI, the inhibitory effect was greatly reduced by the acetylation in the oligosaccharide moiety. However, the reacetylation in the oligosaccharide moiety had no significant change. By analyzing the difference between IX and X, the methylation in the phenolic hydroxyl group dramatically reduced the cytotoxic effect by nearly 50% in the same concentration. In conclusion, further studies are required to investigate whether the flavonol glycosides directly or indirectly, through metabolism, facilitate the development of osteoblasts.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (NSFC) and the Research Grants Council (RGC) of Hong Kong Joint Research Scheme 2004 (No. 30418007, Modern approach to study the osteoprotective effects and active principles of Herba epimedii. and Rhizoma drynariae.).
References
- Aubin J E. Advances in the osteoblast lineage. Biochem Cell Biol 1998; 76: 899–910
- Carmichael J, Degraff W G, Gazdar A F, Minna J D, Mitchell. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 1987; 47: 936–942
- Chen K M, Ge B F, Ma H P, Zheng R L. The serum of rats administered flavonoid extract from Epimedium sagittatum. but not the extract itself enhances the development of rat calvarial osteoblast-like cells in vitro.. Pharmazie 2004; 59: 61–64
- Chen T, Fry D. Hormonal regulation of the osteoblastic phenotype expression in neonatal murine calvarial cells. Calcif Tissue Int 1999; 64: 304–309
- Davison S, Davis S R. Hormone replacement therapy: Current controversies. Clin Endocrinol 2003; 58: 249–261
- Harada S I, Rodan G A. Control of osteoblast function and regulation of bone mass. Nature 2003; 423: 349–355
- Hidaka S, Okamoto Y, Nakajima K. Preventive effects of traditional Chinese medicines on experimental osteoporosis induced by ovariectomy in rats. Calcif Tissue Int 1997; 61: 239–246
- Huang H F, You J S. The use of Chinese herbal medicine on experimental fracture healing. Am J Chin Med 1997; 25: 351–356
- Ji H, Liu K, Gong X J, Li S P, Zhang M F. Effect of Epimedium koreanum. flavonoids on osteoporosis in ovariectomized rats. Chin J Osteoporosis 2001; 7: 4–8
- Jiang Y N, Mo H Y, Chen J M. Effects of Epimedium. total flavonoids phytosomes on preventing and treating bone loss of ovariectomized rats. Chin J Chin Materia Medica 2002; 27: 221–224
- Katzburg S, Lieberherr M, Ornoy A, Klein B Y, Hendel D, Somjen D. Isolation and hormonal responsiveness of primary cultures of human bone-derived cells: gender and age differences. Bone 1999; 25: 667–673
- Li T, Li E, An S. Effect of kidney-tonifying herbs on ovary function and bone mass in postmenopausal women. Chin J Osteoporosis 2000; 6: 55–59
- Liu H C, Chen R M, Jian W C, Lin Y L. Cytotoxic and antioxidant effects of the water extract of the traditional Chinese herb gusuibu (Drunaria fortunei.) on rat osteoblasts. J Formos Med Assoc 2001; 100: 383–388
- Meng F H, Li Y B, Xiong Z L, Jiang Z M, Li F M. Osteoblastic proliferative activity of Epimedium brevicornum. Maxim. Phytomedicine 2005a; 12: 189–193
- Meng F H, Xiong Z L, Jiang Z M, Li F M. Osteoblastic proliferation stimulating activity of Epimedium koreanum. Nakai extracts and its flavonol glycosides. Pharm Biol 2005b; 43: 92–95
- Mizuno M, Sakakibara N, Hanioka S, Iinuma M, Tanaka T, Liu X S, Shi D W. Flavonol glycosides from Epimedium sagittatum.. Phytochemistry 1988; 27: 3641–3643
- Nelson H D, Humphrey L L, Nygren P, Teutsch S M, Allan J D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002; 288: 872–881
- Pinkerton J V, Santen R. Alternatives to the use of estrogen in postmenopausal women. Endocr Rev 1999; 20: 308–320
- Rodriguez J P, Garat S, Gajardo H. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem 1999; 75: 414–423
- Sun P Y, Wen Y, Xu Y, Pei Y P, Chen Y J. The chemical constituents of Epimedium koreanum. Nakai. Acta Pharm Sin 1998; 33: 919–922
- Wlodarski K H. Properties and origin of osteoblasts. Clin Orthop Relat Res 1990; 252: 276–293
- Xie F, Wu C F, Lai W P, Yang X J, Cheung P Y, Yao X S, Leung P C, Wong M S. The osteoprotective effect of Herba epimedii. (HEP) extract in vivo in vitro.. eCAM 2005; 2: 353–361
- Zhang D W, Cheng Y, Wang N L, Zhang J C, Yang M S, Yao X S. Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine 2008; 15: 55–61
- Zhang G, Shi W Z, Shi Y Y. Effect of a compound ‘kidney tonifying’Chinese herbal preparation on femur cortical and trabecular bone loss induced by aging in aged male rats. Chin J Osteoporosis 1999; 5: 51–55