Abstract
Tribulus terrestris. Linn. (Zygophyllaceae) has long been used in the traditional Chinese and Indian systems of medicine for the treatment of various ailments and is popularly claimed to improve sexual function in men. In this study, we have examined the aphrodisiac properties of the furostenol glycoside fraction of Tribulus terrestris. extract (TT-FG) in male castrated rats. Adult Wister rats were castrated and divided into five groups of six animals each and treated with either vehicle, sildenafil (5 mg/kg, p.o.) or TT-FG (5, 10, and 25 mg/kg, p.o.) once daily for 14 days. Sexual behavioral and orientational activity was observed after acute (1 day) and subacute (7 and 14 days) treatment. Serum testosterone levels were also measured on day 14 by withdrawing blood from retroorbital plexus. The improvement in sexual behavior as noted by increase in mounting frequency (MF), intromission frequency (IF), and ejaculation latency (EL) and decrease in mounting latency (ML), intromission latency (IL), and postejaculation interval (PEI) after TT-FG treatment in castrated rats in our study implies increase in the desired component of sexuality. TT-FG was found to increase orientational activity parameters such as licking and ano-and genital grooming and decreased climbing and nongential grooming by male rats indicating increased sexual stimulation and lack of interest toward external environment after treatment with TT-FG. These results are supported by increased serum testosterone levels in male rats after 14 days of treatment by TT-FG. In conclusion, the furostenol glycoside fraction of Tribulus terrestris. exhibited good aphrodisiac properties.
Introduction
Male sexual dysfunction is a common sexual disorder of desire, arousal, orgasm, and sexual pain. It is estimated that about 40 million men suffer from erectile dysfunction, 52% of which are between the ages of 40 and 70 years (Aung et al., Citation2004). Both medical and surgical treatment modalities are available for treating sexual dysfunction. However, despite the increasing availability of effective conventional medical treatments, plant-derived and herbomineral remedies continue to be a popular alternative for men and women seeking to improve their sex lives (Aung et al., Citation2004; Neychev & Mitev, Citation2005).
Tribulus terresteris. Linn (Zygophyllaceae) is distributed up to 11,000 feet altitude throughout India and the warm regions of both hemispheres of the world. In Ayurvedic literature (Kirtikar & Basu, Citation1988), the leaves are reported as aphrodisiac, blood purifier, and tonic, to increase menstrual flow, to cure gonorrhea, and as anti-inflammatory. Fruits are used as diuretic, tonic, aphrodisiac, and are also used in urinary disorders, whereas root is used as a stomachic, appetizer, ammenogogue, diuretic, and carminative. Tribulus terrestris. is widely used as an ingredient in oriental herbal formulations as an energizer and vitalizer to improve sexual function in men (Park et al., Citation2006). Furthermore, Tribulus terrestris. extract has been shown to possess promise toward improvement in sexual activity and erectile function in isolated rat brain studies (Gauthaman & Adaikan, Citation2005) as well as in vivo. aphrodisiac activity in male rats (Park et al., Citation2006) and rabbits (Adaikan et al., Citation2000). Administration of Tribulus terrestris. extract to humans and animals improves libido and spermatogenesis (Tomova et al., Citation1981). Tribulus terrestris. was postulated to act by increasing the release of nitric oxide from the endothelium and nitrergic nerve endings (Adaikan et al., Citation2000). However, the effect on androgen (especially testosterone) is contradictory. In earlier study, the increase in body weight and sexual behavior after Tribulus terrestris. extract administration in rats was postulated to be a secondary effect due to increased androgens (Gauthaman et al., Citation2003), whereas androgen production in young men did not change after treatment with saponins (60% pure) from Tribulus terrestris. extract (Neychev & Mitev, Citation2005).
In order to clarify effects of Tribulus terrestris. on male erectile dysfunction, we have fractionated and separated the steroidal furostenol glycoside fraction from Tribulus terrestris. and evaluated its effects on male erectile dysfunction (i.e., male sexual behavior and testosterone level) in male castrated rats.
Materials and Methods
Chemicals
Testosterone (Himedia Laboratories, Mumbai, India), urethane (Himedia Laboratories), sildenafil citrate (Zydus Cadila Ltd., Ahmedabad, India), estradiol benzoate (Sigma-Aldrich, St. Louis, MO, USA), hydroxyprogesterone (Sigma-Aldrich), and anesthetic ether (TKM Pharma, Pune, India) were purchased from commercial suppliers. All the chemicals were of analytical grade, and solvents were of HPLC grade.
Animals
Wistar rats of weight range 150–200 g of either sex were purchased from National Toxicology Centre (Pune, India) and used for the study. They were maintained at a temperature of 25 ± 1°C and relative humidity of 45% to 55% under 12-h light:12-h dark cycle. The animals had free access to food pellets (Chakan Oil Mills, Pune, India) and water was given ad libitum.. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy (Pune, India), constituted under the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA).
Preparation of furostenol glycoside fraction (TT-FG) of Tribulus terrestris.
Aerial parts of Tribulus terrestris. were collected from forest areas of the western Ghat region of Maharashtra state (India) in the month of September 2005 and were authenticated by A.M. Mujumdar, Department of Botany, Agharkar Research Institute (Pune, India), and a voucher specimen was deposited at that institute.
The crude aerial parts were dried and cut into pieces and washed thoroughly in running water to free the adhering contaminants such as soil, and so forth. This material was dried under shade (30°C, 45% relative humidity). The dried material was then extracted with aqueous ethanol (70%) at room temperature for 8 h in a circulation bed, and the filtrate was made free of suspended foreign matter by decantation. This filtrate was concentrated under vacuum at 45°C to obtain a paste, which was then redissolved in 0.1 M sodium acetate buffer and filtered free of sediment. The clear liquid was loaded on a counter current column (tubular) and extracted with n.-butanol. The butanol layer was separated and concentrated to powder. This powder was dissolved in demineralized water and passed through polymer adsorbent column Amberlite XAD-7 (Rohm & Haas). The column was washed thoroughly with 2% sodium chloride solution followed by demineralized water and eluted in a gradient manner with ethanol:water mixture, and the fractions were collected. The elutants were characterized using high-performance liquid chromatography (HPLC) for furostanol glycosides using CHROMADEX standards. The fractions containing the maximum of these compounds were concentrated under vacuum at 40°C, and the powder was recrystallized in alcohol to get yellow crystals (yield 0.018% w/v).
HPLC details
A Kromosil reverse-phase C-18 column (250 × 4.6 mm with 5-μ m particle size) was used. Mobile phase gradient was (A) water and (B) acetonitrile according to the following profile: at 0 min (75% A and 25% B), at 20 min (65% A and 35% B). Flow rate was 1 mL/min; detector was UV (205 nm).
Acute oral toxicity of drug extract
Acute oral toxicity was carried out in various groups of six male Wistar albino rats (Wistar strain, 120 to 160 g), according to OECD (Organization for Economic Cooperation and Development) guideline no. AOT-425 (2001).
Effect of TT-FG on sexual behavior
The male rats that are capable of mounting over the female rats were selected for this experiment as reported previously (Park et al., Citation2006) and were castrated as described earlier (Ottani et al., Citation2002). The rats were divided into five groups of six animals each. Rats were either treated daily with distilled water (control group) or TT-FG (5, 10, and 25 mg/kg, p.o.) for 14 days. Standard drug, sildenafil citrate (5 mg/kg, p.o.), was administered on observation day only (1 h prior). The ovarectomized female rats were brought to estrus (primed) by sequential administration of estradiol benzoate (10 μ g/kg body weight) and hydroxyprogesterone (1.5 mg/kg body weight), through subcutaneous injections, 48 h and 4 h before the copulatory studies, respectively. Sexual behavior studies were carried out in a separate room under dim-red illumination. The male rat was individually placed in a rectangular Plexiglas chamber, 10 min before the introduction of a primed female, to become acclimatized to the chamber conditions. The primed female was then introduced into the chamber. The TT-FG was administered for 14 days and sexual (copulatory) behavior parameters (Ageel et al., Citation1994; Park et al., Citation2006), such as mounting frequency (MF), intromission frequency (IF), mounting latency (ML), ejaculation latency (EL), intromission latency (IL), postejaculatory interval (PEI), and orientational activities, were observed on the 1st, 7th, and 14th days of drug administration. Copulatory sexual behavior parameters involve MF (the number of mounts without intromission from the time of introduction of the female until ejaculation), IF (the number of intromissions from the time of introduction of the female until ejaculation), ML (the time interval between the introduction of the female and the first mount by the male), IL (interval from the time of introduction of the female to the first intromission by the male and characterized by pelvic thrusting and springing dismount), EL (the time interval between the first intromission and ejaculation characterized by longer, deeper pelvic thrusting and slow dismount followed by a period of inactivity), and PEI (time interval between ejaculation and the first intromission). Orientational activities parameters include behavior of male rats toward the female (licking and anogenital sniffing), male rats toward environment (climbing), and male rats toward self (genital grooming and nongenital grooming). On day 14, blood was withdrawn from each rat from retroorbital plexus, and serum testosterone was estimated using radioimmunoassay (RIA) kits.
Statistical analysis
Mean number of activity was calculated for each behavioral parameter and analyzed by two-way repeated measure ANOVA on ranks followed by post hoc. Holm-Sidak test. p. values < 0.05 were taken as significant.
Results
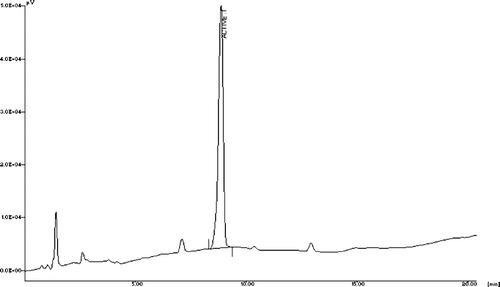
The high-performance liquid chromatogram obtained () from TT-FG showed the presence of one major peak at 9.5 min (protodioscin) and two adjoining minor peaks (neoprotodioscin and protogarcillin).
Effect of TT-FG on sexual behavior of castrated male rats
Two-way repeated measure ANOVA on rank indicated aphrodisiac effect of TT-FG in sexual behavior parameters. The results of Holm-Sidak test indicated that TT-FG (5, 10, and 25 mg/kg, p.o.) as well as sildenafil citrate (5 mg/kg, p.o.) significantly increased the MF, IF, and EL and decreased ML, IL, and PEI after acute administration except the fact that TT-FG at 5 mg/kg, p.o., dose did not increase MF and EL (). On subacute administration, all the treatment groups showed significant (p. < 0.01) increase in MF, IL, and EL and decrease in ML, IL, and PEI after 7 or 14 days of treatment.
Figure 2 Male sexual behavior parameters in male rats after acute (1 day) and subacute (7 and 14 days) oral treatment of TT-FG at (▾) 5 mg/kg, (▪) 10 mg/kg (•), 25 mg/kg. Separate groups for vehicle (▪) and standard drug, sildenafil, 5 mg/kg (▵), were also maintained. Data represented are mean number of observations ± SEM in castrated male rats (six per group) analyzed by two-way repeated measure ANOVA on ranks followed by post hoc. Holm-Sidak test. ns, non-significant compared with values of vehicle-treated group on corresponding day. All other values are significant at p. < 0.05.
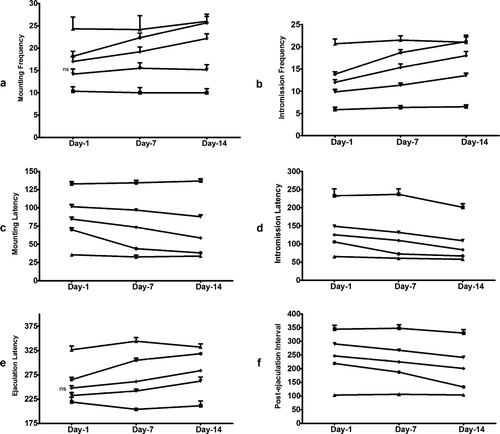
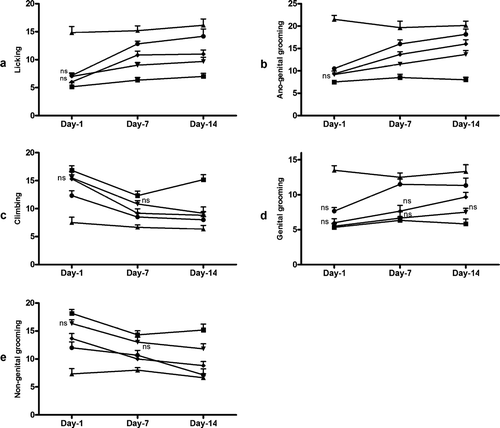
Effect of TT-FG on orientational activities of castrated male rats
Our results () indicated increase in behavioral parameters of orientational activity of male rats by acute administration of TT-FG (10 and 25 mg/kg, p.o.) and sildenafil (5 mg/kg, p.o.) but not by TT-FG (5 mg/kg, p.o.). On subacute administration, all the treatments showed a significant increase in orientational activity after 7 or 14 days of treatment except TT-FG (5 mg/kg, p.o.), which did not change climbing, genital grooming, and nongenital grooming scores after 7 days of treatment ().
Figure 3 Male orientational activity in male rats after acute (1 day) and subacute (7 and 14 days) oral treatment of TT-FG at (▾) 5 mg/kg, (▪) 10 mg/kg, (•) 25 mg/kg. Separate groups for vehicle (▪) and standard drug, sildenafil, 5 mg/kg (▵), were also maintained. Data represented are mean number of observations ± SEM in castrated male rats (six per group) analyzed by two-way repeated measure ANOVA on ranks followed by post hoc. Holm-Sidak test. ns, non-significant compared with values of vehicle-treated group on corresponding day. All other values are significant at p. < 0.05.
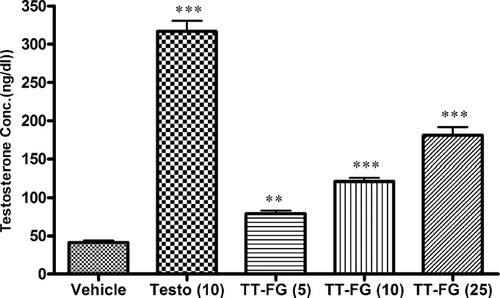
Effect of subacute treatment with TT-FG on serum testosterone levels of castrated male rats
Dose-dependent and significant (p. < 0.001) increase in serum testosterone level was observed after the once-daily treatment with TT-FG (5, 10, or 15 mg/kg, p.o.) for 14 days (). This increase in serum testosterone by TT-FG was comparable with but less than testosterone (10 mg/kg, s.c., biweekly).
Figure 4 Effect of TT-FG on serum testosterone levels in male rats after 14 days of treatment of TT-FG. Separate groups for vehicle and standard drug testosterone were also maintained. Figures in parentheses indicate dose in mg/kg. Data represented are mean testosterone level (ng/dL) ± SEM in castrated male rats (six per group) analyzed by one-way ANOVA followed by Bonferroni post hoc test. **p. < 0.05, ***p. < 0.001 as compared with vehicle-treated group.
Discussion
Erectile dysfunction is the most common cause of male sexual dysfunction (Dinsmore & Evans, Citation1999) and has been shown to compromise overall quality of life. Erectile dysfunction results in depression, anxiety, and loss of self-esteem (Latini et al. Citation2002; Hedon, Citation2003). It may also signal underlying disease including diabetes, hypertension, and cardiovascular disease (Murray et al., Citation1992; Kang et al., Citation2004; Behr-Roussel et al., Citation2005b; Kushiro et al., Citation2005; Lau et al., Citation2005; Papatsoris & Korantzopoulos, Citation2006). The so-called male menopause (andropause) is an old-age disease associated with loss of libido, loss of muscle mass, and decreased testosterone production (Gould & Petty, Citation2000; Margolese, Citation2000; Utiger, Citation2003). Androgen (especially testosterone) replacement has been found to be effective in restoration of these conditions (Heaton & Varrin, Citation1994; Schiavi et al., Citation1997; Yildirim et al., Citation1997; Mulhall et al., Citation2004). Sexual behavior and erection are androgen dependent (acting both centrally and peripherally), and testosterone treatment was reported to restore both sexual behavior and penile erectile capacity in castrated rats (Mills et al. Citation1992, Citation1996; Aversa et al. Citation2000) and in patients (Seftel et al., Citation2004).
In the past, Tribulus terrestris. extract was reported to have a proerectile effect in vitro. against the corpus cavernosal tissues of rabbits with various pharmacological agents and electrical field stimulation (Adaikan et al., Citation2000). Administration of Tribulus terrestris. extract to humans and animals was also reported to improve libido and spermatogenesis (Tomova et al., Citation1981).
In this study, we have made an attempt to investigate the effect of furostenol glycoside fraction isolated from Tribulus terrestris. extract in the castrated rat, which is a convenient model for studying the effect of androgens in relation to sexual characteristics (Mills et al., Citation1996). Castration leads to low androgenic status affecting structural, biochemical, pharmacological, or any of these components of erectile physiology, which in turn causes reduction in erectile function as observed from studies on various animal models (Hart et al., Citation1983). Physiologically, low levels of androgen as seen in hypogonadism is associated with decreased sexual desire and activity (Rabkin et al. Citation1999; Morales & Heaton, Citation2001). Apart from desire that is essential for initiation of sex, penile tumescence and rigidity as well as the accessory muscles that help in providing additional penile rigidity and ejaculation are also dependent on androgen for normal sexual activity. Various neurotransmitters and their inter/intracellular signaling are responsible for the relaxation of corpus cavernosal smooth muscle (CCSM). Although the exact mechanism of action is still to be elucidated (Heaton & Varrin, Citation1994), androgens influence these neurotransmitters and endogenous substances like nitric oxide (Chamness et al., Citation1995) and contribute to the regulation of penile erection.
The improvement in sexual behavior as noted by increase in MF, IL, and EL and decrease in ML, IL, and PEI after TT-FG administration in castrated rats in our study implies increase in the desired component of sexuality, and these results correlate well with that of testosterone replacement therapy (Gauthaman et al., Citation2002). Sildenafil improved sexual performance, which is in agreement with earlier reports of improvement in sexual performance in castrated rats by sildenafil (Giuliani et al., Citation2002; Ottani et al., Citation2002; Hadidi et al., Citation2003), although the effects of TT-FG are less than those of sildenafil. Our study is also supported by the earlier reports of proerectile effects of chronic sildenafil treatment (Gemalmaz et al., Citation2001; Giuliano et al., Citation2003; Behr-Roussel et al., Citation2005a). In our study, TT-FG was found to increase orientational activity parameters such as licking and anogenital grooming of male rats () indicating increased sexual stimulation of male rats toward females after treatment. Genital grooming plays a major role in the readiness of adult male rats for reproduction (Hernandez-Gonzalez, Citation2000) and is an important indicator of erectile status of the penis (Rampin et al., Citation2003). These results are further supported by the decrease in climbing and nongential grooming responses shown by male rats in our study and indicate lack of interest shown by male rats toward external environment in favor of females. Furthermore, serum testosterone levels in male rats were found to increase after 14 days of treatment by TT-FG (). The continued administration of TT-FG for 2 weeks in this study might have increased the androgenic status both by central and peripheral mechanisms. The presence of protodioscine, the probable active ingredient of TT-FG (, HPLC), is reported to improve libido and spermatogenesis in humans and animals (Tomova et al., Citation1981) and to increase the levels of testosterone, luteinizing hormone (Protich et al., Citation1983), and dehydroepiandrosterone (DHEA) (Adimoelja & Adaikan, Citation1997; Adimoelja, Citation2000). DHEA, which is a major circulating steroid in human plasma, is a common precursor for both androgens and estrogens (Miller, Citation1988). DHEA also acts centrally as a GABA antagonist to facilitate sexual function (Majewska, Citation1995). Possible increase in DHEA and its subsequent conversion to testosterone and its metabolites may account for the increased testosterone level and observed behavioral effects in this study. Our work confirmed the effect of TT-FG of which protodioscin is a major constituent (Gauthaman et al., Citation2002). Significant increase in serum testosterone level may be due to conversion of protodioscin (present in TT-FG) to testosterone.
It is thus apparent that the furostenol glycoside fraction of Tribulus terrestris. has potential as a remedy for the treatment of male erectile dysfunction. Results of this study may also act as proof of concept that Tribulus terrestris. as an aphrodisiac as reported by traditional medicine.
References
- Adaikan P G, Gauthaman K, Prasad R N, Ng S C. Proerectile pharmacological effects of Tribulus terrestris. extract on the rabbit corpus cavernosum. Ann Acad Med Singapore 2000; 29: 22–26
- Adimoelja A. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int J Androl 2000; 23: 82–84, (Suppl 2)
- Adimoelja A, Adaikan P G. Protodioscin from herbal plant Tribulus terrestris. L. improves male sexual functions possibly via DHEA. Int J Impot Res 1997; 9: S64
- Ageel A M, Islam M W, Ginawi O T, Al-Yahya M A. Evaluation of the aphrodisiac activity of Litsea chinesis. (Lauraceae) and Orchis maculate. (Orchidaceae) extracts in rats. Phytotherapy 1994; 8: 103–105
- Aung H H, Dey L, Rand V, Yuan C S. Alternative therapies for male and female sexual dysfunction. Am J Chin Med 2004; 32: 161–173
- Aversa A, Isidori A M, De Martino M U, Caprio M, Fabbrini E, Rocchietti-March M, Frajese G, Fabbri A. Androgens and penile erection: Evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol (Oxf) 2000; 53: 517–522
- Behr-Roussel D, Gorny D, Mevel K, Caisey S, Bernabe J, Burgess G, Wayman C, Alexandre L, Giuliano F. Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: Lack of tachyphylaxis. Eur Urol 2005a; 47: 87–91
- Behr-Roussel D, Gorny D, Mevel K, Compagnie S, Kern P, Sivan V, Bernabe J, Bedigian M P, Alexandre L, Giuliano F. Erectile dysfunction: An early marker for hypertension? A longitudinal study in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 2005b; 288: R276–283
- Chamness S L, Ricker D D, Crone J K, Dembeck C L, Maguire M P, Burnett A L, Chang T S. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril 1995; 63: 1101–1107
- Dinsmore W, Evans C. ABC of sexual health: Erectile dysfunction. BMJ 1999; 318: 387–390
- Gauthaman K, Adaikan P G. Effect of Tribulus terrestris. on nicotinamide adenine dinucleotide phosphate-diaphorase activity and androgen receptors in rat brain. J Ethnopharmacol 2005; 96: 127–132
- Gauthaman K, Adaikan P G, Prasad R N. Aphrodisiac properties of Tribulus terrestris. extract (protodioscin) in normal and castrated rats. Life Sci 2002; 71: 1385–1396
- Gauthaman K, Ganesan A P, Prasad R N. Sexual effects of puncturevine (Tribulus terrestris.) extract (protodioscin): An evaluation using a rat model. J Altern Complement Med 2003; 9: 257–265
- Gemalmaz H, Waldeck K, Chapman T N, Tuttle J B, Steers W D, Andersson K E. In vivo in vitro. investigation of the effects of sildenafil on rat cavernous smooth muscle. J Urol 2001; 165: 1010–1014
- Giuliani D, Ottani A, Ferrari F. Influence of sildenafil on copulatory behavior in sluggish or normal ejaculator male rats: A central dopamine mediated effect?. Neuropharmacology 2002; 42: 562–567
- Giuliano F, Bernabe J, Alexandre L, Niewoehner U, Haning H, Bischoff E. Pro-erectile effect of vardenafil: In vitro. experiments in rabbits and in vivo. comparison with sildenafil in rats. Eur Urol 2003; 44: 731–736
- Gould D C, Petty R. The male menopause: Does it exist?. West J Med 2000; 173: 76–78
- Hadidi K A, Aburjai T, Battah A K. A comparative study of Ferula hermonis. root extracts and sildenafil on copulatory behavior of male rats. Fitoterapia 2003; 74: 242–246
- Hart B L, Wallach S J, Melese-d'Hospital P Y. Differences in responsiveness to testosterone of penile reflexes and copulatory behavior of male rats. Horm Behav 1983; 17: 274–283
- Heaton J P, Varrin S J. Effects of castration and exogenous testosterone supplementation in an animal model of penile erection. J Urol 1994; 151: 797–800
- Hedon F. Anxiety and erectile dysfunction: A global approach to ED enhances results and quality of life. Int J Impot Res 2003; 15: S16–19, (Suppl 2)
- Hernandez-Gonzalez M. Prepubertal genital grooming and penile erections in relation to sexual behavior of rats. Physiol Behav 2000; 71: 51–56
- Kang K K, Choi S M, Ahn G J, Kwon J W, Kim W B. The effect of DA-8159 on corpus cavernosal smooth muscle relaxation and penile erection in diabetic rabbits. Urol Res 2004; 32: 107–111
- Kirtikar K R, Basu B D. Indian Medicinal Plants, 3rd ed. International Book Distributor, Dehra Dun 1988; 1126–1127
- Kushiro T, Takahashi A, Saito F, Otsuka Y, Soma M, Kurihara T, Satomura A, Saito T, Kanmatsuse K. Erectile dysfunction and its influence on quality of life in patients with essential hypertension. Am J Hypertens 2005; 18: 427–430
- Latini D M, Penson D F, Colwell H H, Lubeck D P, Mehta S S, Henning J M, Lue T F. Psychological impact of erectile dysfunction: Validation of a new health related quality of life measure for patients with erectile dysfunction. J Urol 2002; 168: 2086–2091
- Lau D H, Kommu S, Mikhailidis D P, Morgan R J, Mumtaz F H. Regarding the prevalence of hypertension, hyperlipidemia, diabetes mellitus and depression in men with erectile dysfunction. J Urol 2005; 173: 1050
- Majewska M D. Neuronal actions dehydroepiandrosterone. Dehydroepiandrosterone (DHEA) and Aging, F L Bellino, R A Daynes, P J Hornsby, D H Lavrin, J E Nestler. New York Academy of Sciences, New York 1995; 111–120
- Margolese H C. The male menopause and mood: Testosterone decline and depression in the aging male—is there a link?. J Geriatr Psychiatry Neurol 2000; 13: 93–101
- Miller W L. Molecular biology of steroid hormone synthesis. Endocr Rev 1988; 9: 295–318
- Mills T M, Reilly C M, Lewis R W. Androgens and penile erection: A review. J Androl 1996; 17: 633–638
- Mills T M, Wiedmeier V T, Stopper V S. Androgen maintenance of erectile function in the rat penis. Biol Reprod 1992; 46: 342–348
- Morales A, Heaton J P. Hormonal erectile dysfunction. Evaluation and management. Urol Clin North Am 2001; 28: 279–288
- Mulhall J P, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63: 348–352, discussion 352–353
- Murray F T, Johnson R D, Sciadini M, Katovich M J, Rountree J, Jewett H. Erectile and copulatory dysfunction in chronically diabetic BB/WOR rats. Am J Physiol 1992; 263: E151–157
- Neychev V K, Mitev V I. The aphrodisiac herb Tribulus terrestris. does not influence the androgen production in young men. J Ethnopharmacol 2005; 101: 319–323
- OECD (Organization for Economic Co-operation and Development). Guidance Document on Acute Oral Toxicity Testing. Environment Directorate, OECD, Paris 2001; 1–24
- Ottani A, Giuliani D, Ferrari F. Modulatory activity of sildenafil on copulatory behavior of both intact and castrated male rats. Pharmacol Biochem Behav 2002; 72: 717–722
- Papatsoris A G, Korantzopoulos P G. Hypertension, antihypertensive therapy, and erectile dysfunction. Angiology 2006; 57: 47–52
- Park S W, Lee C H, Shin D H, Bang N S, Lee S M. Effect of SA1, a herbal formulation, on sexual behavior and penile erection. Biol Pharm Bull 2006; 29: 1383–1386
- Protich M, Tsvetkov D, Nalbanski B, Stanislavov R, Katsarova M. [Clinical trial of a tribestan preparation in infertile men]. Akush Ginekol (Sofiia) 1983; 22: 326–329
- Rabkin J G, Wagner G J, Rabkin R. Testosterone therapy for human immunodeficiency virus-positive men with and without hypogonadism. J Clin Psychopharmacol 1999; 19: 19–27
- Rampin O, Jerome N, Suaudeau C. Proerectile effects of apomorphine in mice. Life Sci 2003; 72: 2329–2336
- Schiavi R C, White D, Mandeli J, Levine A C. Effect of testosterone administration on sexual behavior and mood in men with erectile dysfunction. Arch Sex Behav 1997; 26: 231–241
- Seftel A D, Mack R J, Secrest A R, Smith T M. Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. J Androl 2004; 25: 963–972
- Tomova M, Gjulemetova R, Zarkova S, Peeva S, Pangarova T, Simova M. Steroidal saponins from Tribulus terrestris L. with a stimulating action on the sexual functions. International Conference of Chemistry and Biotechnology of Biologically Active Natural Products. RSC Publishing, Varna, Bulgaria 1981; 298–302
- Utiger R D. Testosterone “fix”: Youth or consequences? Should we treat “male menopause”?. Health News 2003; 9: 3
- Yildirim M K, Yildirim S, Utkan T, Sarioglu Y, Yalman Y. Effects of castration on adrenergic, cholinergic and nonadrenergic, noncholinergic responses of isolated corpus cavernosum from rabbit. Br J Urol 1997; 79: 964–70