Abstract
The current study evaluated the antioxidant and hepatoprotective activities of Elephantopus tomentosus. L. (Asteraceae) ethanol extract (ET). In the experiment, total antioxidant capacity, reducing capacity, DPPH* and hydrogen peroxide scavenging, and Fe3 +-induced lipid peroxidation inhibiting activities of ET were determined. The results indicated that ET exhibited antioxidant (1 mg/mL ET was equal to 2.1 mM TEAC), lipid peroxidation inhibition, hydrogen peroxide, and free radical scavenging activities. The hepatoprotective activity of ET was studied using CCl4-induced liver toxicity in rats. Oral administration of ET (500 mg/kg) significantly reduced CCl4-induced hepatotoxicity in rats, as observed from the serum level of the liver enzyme aspartate aminotransferase (AST), alanine aminotransferase (ALT), and also from the histopathologic study. The total phenolic content in the lyophilized ethanol extract is approximately 10%. The results of the current study indicated that the hepatoprotective effect of E. tomentosus. might be ascribable to its antioxidant and free radical scavenging properties.
Introduction
Liver disease remains one of the most serious health problems. Unfortunately, current conventional or synthetic drugs used in the treatment of liver disease are inadequate and sometimes cause serious side effects. This is one of the reasons many people around the world prefer traditional remedies for the treatment of liver ailments. Therefore, it is necessary to search for alternative drugs for the treatment of liver disease for more efficacy and safety to replace currently used drugs (Mitra et al., Citation2000; Rao et al., Citation2006).
Oxidation processes are very important for living organisms. The uncontrolled production of oxygen free radicals and the unbalanced mechanism of antioxidant protection result in the onset of many diseases, namely cancer, diabetes, Alzheimer's disease, coronary heart diseases and aging (Hertog et al., Citation1995; Keli et al., Citation1996; Deng et al., Citation1998; Geleijnse et al., Citation1999, Citation2000; Erlund et al., Citation2001; Yang et al., Citation2004). Antioxidants are considered possible protective agents reducing oxidative damage to the human body. Therefore, there is a growing interest in the substances exhibiting antioxidant properties that are supplied to human and animal organisms as food components or as specific pharmaceuticals. There are two basic categories of antioxidant; namely, the synthetic and the natural antioxidant. Restriction on the use of synthetic antioxidants is being imposed due to their carcinogenicity (Grice, Citation1986, Citation1988). Recently, natural antioxidants have become one of the major areas of scientific research (Demo et al., Citation1998; Sanchez-Moreno et al., Citation1999). Owing to safety concerns of synthetic antioxidants and effectiveness of natural antioxidants, the public prefers to take natural antioxidants from edible materials such as fruits, spices, herbs and vegetables. Therefore, the development and use of more effective antioxidants of natural origin is desired.
Members of the Elephantopus. (Asteraceae) genus are widely used in South East Asian folk medicine. Elephantopus scaber. Linn. is believed to possess antipyretic, anti-inflammatory, and emollient activities. In Malaysia, E. tomentosus. L. is taken internally as a diuretic, febrifuge, analgesic, anthelmintic, and anti-inflammatory agent. E. tomentosus. is also applied externally as a poultice for abdominal pains. However, little scientific study of E. tomentosus. has been made. Recently, E. scaber. and E. mollis. were found to have a liver protective effect on several kinds of chemically induced liver damage, namely carbon tetrachloride (CCL4), β-d-galactosamine (d-GalN), and acetaminophen (APAP) (Lin et al., Citation1991, Citation1995). Other studies also found that E. scaber. water extract is effective as an anti-inflammatory agent (Tsai & Lin, Citation1999).
No documented reports are available so far on the evaluation of this plant for possible antioxidant and hepatoprotective activities. Therefore, to justify the traditional claims, we have assessed the hepatoprotective effects of E. tomentosus. using CCl4-induced hepatoxicity in vivo. and lipid peroxidation in vitro. as experimental models. To elucidate the antioxidant properties of E. tomentosus., in vitro. experiments on Trolox equivalent antioxidant capacity test, reducing capacity, as well as hydrogen peroxide and 2,2′-diphenyl-1-picrylhydrazyl scavenging activities were conducted. Total phenolic content was also determined because previous studies showed that phenolic compounds played an important role in antioxidant and hepatoprotective activities.
Materials and Methods
Chemicals
Folin-Ciocalteu reagent, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), sodium carbonate (Na2CO3), 2,2′azino-bis.(3-ethy)benz-thiazoline-6-sulfonic acid (ABTS), butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), potassium persulfate, phosphate-buffered saline (PBS), gallic acid, trichloroacetic acid (TCA), ferric chloride (FeCl3), and Harris hematoxylin were purchased from Sigma (St. Louis, MO, USA). Potassium chloride (KCl) and carboxymethylcellulose (CMC) were purchased from BDH Chemicals Ltd. (Poole, England). Potassium ferricyanide [K3Fe(CN)6] and hydrochloric acid (HCl) were purchased from R&M marketing (Essex, UK). Methanol, absolute alcohol, and eosin were purchased from Riedel-de Haën (Seezle, Germany). Hydrogen peroxide (H2O2) and xylene were purchased from Fisher Scientific (Leics, UK). Trolox was purchased from Calbiochem (Darmstadt, Germany). Thiobarbituric acid (TBA) was purchased from Applichem (Darmstadt, Germany). Paraplast was purchased from Oxford Labware (St. Louis, MO, USA). Disposable microtome blades 818 were purchased from LEICA (Germany).
Preparation of plant extract
The plants were collected from Permatang Damar Laut, Penang, Malaysia, and sent to the Herbarium School of Biological Sciences, Universiti Sains Malaysia, for identification. The herbarium voucher number is 10832. After identification, the plant was dried in an oven at 45°C. The plant was then ground into powder and extracted with 95% ethanol in a Soxhlet apparatus. The extract was concentrated using a rotary evaporator (Büchi RE121, Switzerland) under vacuum and then dried in a freeze-dryer (HETO Hetovac VR-1, Birkerød, Denmark). The lyophilized ethanol extract of E. tomentosus. (ET) was then kept in desiccators at room temperature prior to the experiments.
Experimental animals
Male Sprague-Dawley (SD) rats (180–220 g) were used for the experiments. The animals were kept under standard conditions (26–30°C) with free access to food and water. All the work involving animals was carried out under the regulations of the university's animal ethics committee.
Assessment of total antioxidant activity
The total antioxidant activity (TAA) values were estimated by the Trolox equivalent antioxidant capacity (TEAC) test (Re et al., Citation1999). ABTS was dissolved in deionized water to give a 7 mM solution. ABTS radical cation (ABTS· +) was produced by reacting the ABTS stock solution with 2.45 mM potassium persulfate. The mixture was allowed to stand in the dark at room temperature (22–24°C) for 12–16 h before use. The concentrated ABTS+ solution was diluted with PBS, pH 7.4, to a final absorbance of 0.70 ± 0.02 at 734 nm at 30°C. Stock solutions of Trolox (0.5–4 mM) and ET (0.5 mg/mL) were prepared in deionized water. The spectrophotometer (Hitachi U-2000, Japan) was preliminarily blanked with PBS. Ten microliters of antioxidant-containing solution were added to 2 mL of ABTS+ solution. The decrease in absorbance was measured at 734 nm, 6 min after addition of Trolox and ET. All sample determinations were carried out in triplicate. The TEAC of ET was calculated by relating the decrease in absorbance to that of Trolox solution on a molar basis.
Measurement of reducing capacity
Reducing capacity was determined by the method of Oyaizu (Citation1986). One milliter of different concentrations of ET, BHA, and BHT (0.5, 0.25, 0.125, 0.0625 mg/mL) (in methanol) was mixed with phosphate buffer (2.5 mL, 0.2 mol/L, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm (using Eppendorf 5403; Engelsdorf, Germany) for 10 min. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm (Hitachi U-2000). Increased absorbance of the reaction indicated increased reducing power.
Assessment of lipid peroxidation inhibition
Adult male Sprague-Dawley rats (180–220 g) were used in this study. The animals were terminally anesthetized with diethyl ether. The liver was removed via abdominal dissection, and 5% (w/v) homogenate of the liver in 0.15 M KCl was prepared using homogenizer (Homogeniser MSE, England) under ice-cold (0–4°C) conditions. The homogenate was then centrifuged (using Eppendorf 5403) at 3000 rpm for 15 min at 4°C, and the resultant supernatant was separated for analysis. First, 100 μ L ET (0.2, 0.4, 0.6, 0.8, 1.0 mg/ml in 70% ethanol) was dispensed into different test tubes, and then followed by 0.5 mL supernatant and 1 mL 0.15 M KCl. Peroxidation was initiated by the addition of 100 μ L 0.2 mM FeCl3. The mixture was incubated at 37°C for 30 min after which the reaction was stopped by adding 2 mL ice-cold TBA-TCA-HCl-BHT solution. The TBA-TCA-HCl was prepared by dissolving 1.68 g TCA and 41.60 mg TBA in 10 mL 0.125 M HCl. One milliter BHT solution (1.5 mg/mL ethanol) was added to 10 mL TBA-TCA-HCl solution. The reaction mixture was heated for 60 minutes at 90°C and then cooled on ice and centrifuged at 3000 rpm. Supernatants were removed, and the color absorbance was measured on a spectrophotometer at 532 nm (using Hitachi U-2000) (Murthy et al., Citation2002; Arts et al., Citation2003). A control experiment was performed in the presence of 70% ethanol without ET. The percentage inhibition of lipid peroxidation in the samples was calculated using the following formula:
where A.0 is the absorbance of the control (100 μ L 70% ethanol), and A.1 is the absorbance of sample containing ET.
Hydrogen peroxide scavenging activity
Hydrogen peroxide scavenging activity of ET was determined according to the method described by Oktay et al. (Citation2003). Two millimolar (mM) solution of hydrogen peroxide was prepared in 0.1 M phosphate buffer (pH 7.4). Then, 300 μ L ET and BHT (0.032, 0.0625, 0.125, 0.25, 0.5, 1.0 mg/mL) dissolved in 70% ethanol was mixed with 180 μ L of the 2 mM hydrogen peroxide solution. The mixture was incubated for 10 min, and absorbance was then determined using a spectrophotometer (Hitachi U-2000) at 230 nm. For each concentration of the extract, a separate blank sample was used for background without hydrogen peroxide. The percentages of hydrogen peroxide scavenging of the extracts were calculated according to the following formula:
where A.0 is the absorbance of the control (180 μ L 2 mM hydrogen peroxide solution mixed with 300 μ L 70% ethanol), and A.1 is the absorbance of the sample containing E. tomentosus. extract. The hydrogen peroxide scavenging activity of ET and BHT were expressed as EC50. The EC50 value was defined as concentration (in mg/mL) of sample that scavenges 50% of the hydrogen peroxide.
DPPH Scavenging activity
A modified Hatano et al. (Citation1989) method was used in the experiment. Firstly, 2 mL of 0.1 mM DPPH (in methanol) was mixed with l00 μ L of various concentrations (0.032, 0.0625, 0.125, 0.25, 0.5, 1.0 mg/mL) of ET, rutin, or quercetin. After 30 min incubation at room temperature (24–26°C), the absorbances of the mixture in the samples were measured using a spectrophotometer (Hitachi U-2000) at 517 nm against methanol as blank. The percentage radical scavenging activity of the samples was evaluated by comparing with a control (2 mL DPPH solution and 100 μ L methanol). Each sample was measured in triplicate and averaged. The radical scavenging activity (RSA) was calculated using the following formula:
where A.0 is the absorbance of the control, and A.1 is the absorbance of samples after 30 min. The free radical scavenging activities of ET, rutin, and quercetin were expressed as EC50. The EC50 value was defined as concentration (in mg/mL) of sample that inhibits 50% of the formation of DPPH radical.
Hepatoprotection study
CCl4-induced liver damage
Rats were divided into three groups of six rats each. Groups A and B were administered 1% of CMC 10 mL/kg (p.o.). Group C was given 500 mg/kg of ET (p.o.). The vehicle or extract was administered orally for 7 days. CCl4 diluted with olive oil (1:1) was administered as 1 mL/kg orally to groups B and C. Twenty four hours after CCl4 administration, blood was collected from all groups by cardiac puncture, and serum was separated by centrifugation at 3500 rpm (using Eppendorf 5403) at 4°C for 15 min and analyzed for various biochemical parameters.
Assessment of liver function
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were analyzed at the Department of Biochemistry, Pathology Laboratory, Lam Wah Ee Hospital (Penang, Malaysia) using Automatic Analyzer 912 (Hitachi).
Liver histopathologic assessment
Immediately after sacrifice of rats, the livers were removed and fixed in 10% formalin. The tissues were fixed in 10% formalin before being processed using histokinette (Citadel 1000, Frankfurt, Germany). After processing, the tissues were embedded in paraffin with Histo-Center II-N (Barnstead/Thermolyne, Iowa, USA) and sectioned into 5-μ m thickness using Histocut 820 (Reichert-Jung, Germany). The sections were stained with hematoxylin and eosin (H&E) for microscopic observation, which includes cell necrosis, fatty changes, and infiltration of Kupffer cells and lymphocytes.
Determination of total phenolic content of ET
The concentration of total phenol in the extract (0.5 mg/mL) was determined using Folin-Ciocalteu phenol reagent and external calibration with gallic acid (Slinkard & Singleton, Citation1977). One hundred microliters extract solution followed by 2 mL distilled water was dispensed into a test tube and 0.2 mL Folin-Ciocalteu reagent was added and mixed thoroughly. After 4 min, 1 mL 15% Na2CO3 was added, and the mixture was allowed to stand for 2 h at room temperature. The absorbance was then measured using a spectrophotometer (Hitachi U-2000) at 760 nm. The concentration of the total phenolic content was determined as mg/mL of gallic acid equivalent (GAE) by using an equation obtained from gallic acid calibration curve y. = 1.5941 × −0.0194, where y. is the unit absorbance and x. is the concentration of phenolic compound (GAE). The coefficient of determination (R2) of the standard curve was 0.993.
Statistical analysis
Statistical analysis involved the use of the Statistical Package for Social Sciences (SPSS) program. Data were indicated as the mean ± SEM and were determined statistically using two-way analysis of variance (ANOVA). Significant differences between the means of groups were determined using LSD multiple comparison test, and P. < 0.05 was taken as significant.
Results and Discussion
Trolox equivalent antioxidant capacity
The reaction of ET with ABTS radicals was examined. The TEAC assay is applied to assess the total amount of ABTS radical that can be scavenged by ET. The TEAC of (1 mg/mL) ET at 6 min was 2.1 ± 0.05 mM.
Lipid peroxidation inhibition
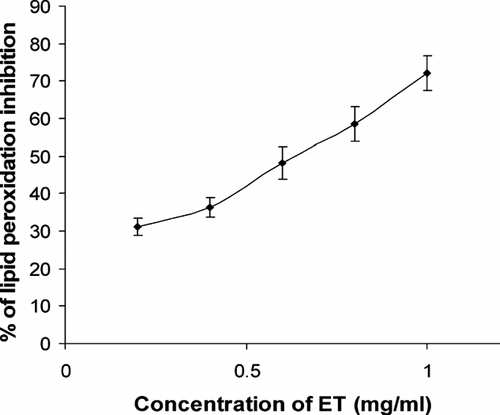
Lipid peroxidation is a natural metabolic process under normal conditions. Phospholipids present in the cellular membrane are the primary substrates of lipid oxidation. Polyunsaturated fatty acids (PUFAs) present in the supernatant of liver homogenate will undergo catalysis by iron ion to form lipid alkoxyl radical (PUFA-O·) under acidic conditions (Liu, Citation1970). This PUFA-O· will cause damage in the human body if present in excess. Results from this study showed that ET is an inhibitor of lipid peroxidation (). The percentage inhibition of the lipid peroxidation of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/ml extracts were 31.2 ± 2.2%, 36.3 ± 2.6%, 48.3 ± 4.3%, 58.7 ± 4.6%, and 72.2 ± 4.6%, respectively.
Hydrogen peroxide scavenging activity
Hydrogen peroxide occurs naturally at low concentration levels in the air, water, human body, plants, microorganisms, food, and beverages. It is widely used as a bleaching agent in the textile, paper, and pulp industries. Human exposure to H2O2 indirectly via the environment is estimated as 0.28 mg/kg a day with intake from leaf crops contributing most to this exposure. Hydrogen peroxide enters the human body through inhalation of the vapor or mist and through eye or skin contact. In the body, H2O2 is rapidly decomposed into oxygen and water, and this may produce hydroxyl radicals (OH·) that can initiate lipid peroxidation and cause DNA damage. ET contains nearly 10% phenolic compounds that are good electron donors and may accelerate the conversion of H2O2 to H2O (Rosen et al., Citation1984). The EC50 values of H2O2 scavenging activity of ET and BHT were 0.25 ± 0.02 and 0.06 ± 0.001 mg/mL, respectively.
Reducing capacity
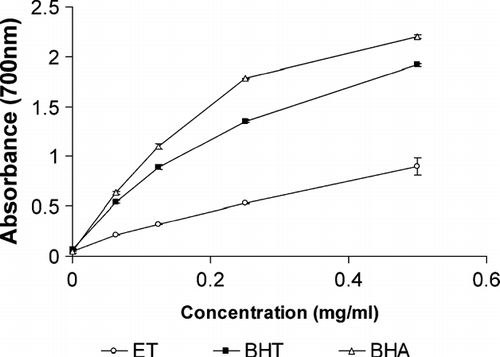
shows the reducing capabilities of ET compared with BHA and BHT. The reducing capacity of an extract or compound may serve as a significant indicator of its potential antioxidant activity (Meir et al., Citation1995). Reducing power of ET and standard compounds followed the order BHA > BHT > ET.
DPPH Scavenging activity
DPPH is a stable free radical that accepts an electron or hydrogen to form a stable diamagnetic molecule. DPPH, which is purple in methanol, will turn into a yellow solution in the presence of a free radical scavenging agent (Molyneux, Citation2004). In the current study, ET is shown to serve as an antioxidant agent or hydrogen donor that can scavenge free radical. It is visually noticeable as a change of methanol DPPH from purple to yellow. The scavenging effect of ET increased with increasing concentration of the extracts. The EC50 values of ET, rutin and quercetin were 0.8867 ± 0.022, 0.126 ± 0.002, and 0.08 ± 0.002 mg/mL, respectively.
Hepatoprotection study
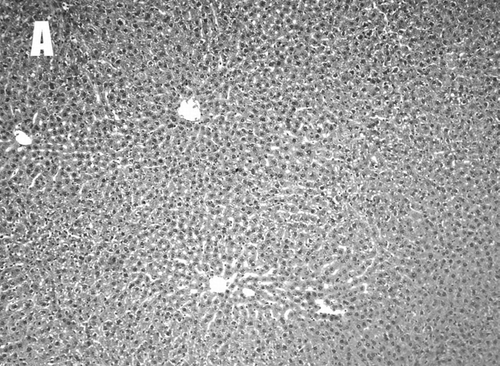
Liver sections of normal control rats showed normal hepatic cells with well-preserved cytoplasm, prominent nuclei, and nucleoli and well brought out central veins (). CCl4 administration induced centrilobular necrosis, and hepatic cells surrounding the central vein showed various degenerative changes like cloudy swelling, extensive infiltration of lymphocytes and Kupffer cells, and necrosis with loss of nuclei and nucleoli. Large cells surrounding the necrotic zone are ballooned hepatocytes, and this may be due to macrovesicular steatosis (). Extent of necrosis bridges to other centrilobular veins are also observed in the CMC plus CCl4–treated group. Pretreatment of the rats with 500 mg/kg ET showed protection against CCl4-induced liver damage (). Liver necrosis was limited to cells around the centrilobular veins and extension of necrosis bridges was not observed; there was less infiltration of Kupffer cells and lymphocytes ().
Figure 3 Histopathology of normal, CMC plus CCl4, and CCl4 plus ET treated rat liver section (× 100, H&E). Liver section of control rats showed normal hepatic cells with well preserved cytoplasm, prominent nuclei, and nucleoli, and well brought out central vein (A). CMC plus CCl4-treated group showed centrilobular necrosis and broad infiltration of lymphocytes and Kupffer cells were observed. Extension of necrosis bridges to other centrilobular veins were also found in the CMC plus CCl4-treated group (B). Pretreatment of the rats with 500 mg/kg ET showed protection to CCl4-induced liver damage. Liver necrosis was limited to cells around centrilobular veins without the present of necrosis bridges (C).
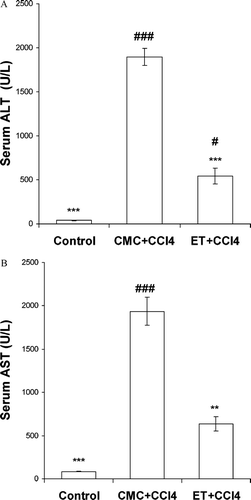
Results of biochemical tests showed a reduction in the levels of ALT and AST in the ET plus CCl4 group when compared with the CCl4 group (). In the ET plus CCl4 group, the levels of ALT and AST are statistically significantly lower (p < 0.001 and p < 0.01, respectively) compared with the CMC plus CCl4–pretreated group (). Compared with the control group, the ET plus CCl4 group showed no significant increase (p < 0.05) for AST ().
Figure 4 (A) Effect of different doses of ET on serum ALT level elevation by CCl4 (n = 6). Values are means ± SEM; *** indicates significant difference compared with the CMC plus CCl4-treated group at p < 0.05 and p < 0.01, respectively; # and ### indicate significant differences compared with the control group at p < 0.05 and p < 0.001 respectively. (B) Effect of different doses of ET on serum AST level elevation by CCl4 (n = 6). Values are means ± SEM; ** and *** indicate significant differences compared with the CMC plus CCl4 group at p < 0.01 and p < 0.001, respectively; ### indicates significant difference compared with the control group at p < 0.001.
CCl4 induces destruction of microsomal cytochrome P-450 and generation of radicals such as trichloromethyl or trichloromethylperoxy radicals that cause lipid peroxidation (Pasquali-Ronchetti et al., Citation1980; De Groot & Hass, Citation1981; Shen et al., Citation1982). These radicals are capable of initiating a chain of lipid peroxidation reactions by abstracting hydrogen from PUFA (Gosselin et al., Citation1984). Peroxidation of lipids can dramatically change the properties of biological membrane, resulting in severe cell damage (Lieber, Citation1988). Therefore, possible hepatoprotective mechanisms of ET may be due to lipid peroxidation inhibition, antioxidation and free radical scavenging effects (Yam et al., Citation2007).
Total phenolic compounds
The phenolic compounds are rich in hydroxyl groups and believed to have the ability for scavenging and stabilizing lipid oxidation (Hatano et al., Citation1989; Yen et al., Citation1993). The total phenolic content of ET is approximately 10%. The phenolic compounds are thought to contribute directly to antioxidant activity (Duh et al., Citation1999), and some studies suggest a correlation between phenolic content and antioxidant and hepatoprotective activities (Yen et al., Citation1993; Yang et al., Citation2002; Yoshikawa et al., Citation2002). Phenolic compounds also exhibit a wide spectrum of biochemical activities including inhibition of xanthine oxidase, glutathione reductase, NADPH oxidase, lipoxygenase, protein kinase C, cyclic-AMP phosphodiesterase, phospholipase A2, calcium-dependent ATPase, oxidation (radical scavenging and metal ion chelation) and cytotoxic effects of oxidized LDL (Beretz et al.,Citation1978; Chang et al., Citation1994; Da Silva et al., Citation1998; Elliot et al., Citation1992; Ferriola et al., Citation1989; Fewtrell & Gompert, Citation1977; Lindhal & Tagesson, Citation1993; Rice-Evans et al., Citation1995; Rosenblat et al., Citation1999).
In conclusion, the ethanol extract of Elephantopus tomentosus. showed strong antioxidant activity, reducing power, lipid peroxidation inhibition, DPPH radical scavenging, hydrogen peroxide scavenging, and hepatoprotective activities. The results of this study show that the ethanol extract of ET can be used as a natural antioxidant and as a possible food supplement. However, the polyphenolic compounds or other components responsible for antioxidant and hepatoprotective effect are still unknown. Therefore, it is suggested that further work should be performed on the isolation and identification of the antioxidant and hepatoprotective components in ET.
Acknowledgment
The authors are grateful to Mr. Yap Tor Hor for critically reviewing the manuscript.
References
- Arts M JTJ, Dallinga J S, Voss H P, Haenen G RMM, Bast A. A critical appraisal of the use of the antioxidant capacity (TEAC) assay in defining optimal antioxidant structures. Food Chem 2003; 80: 409–414
- Beretz A, Anton R, Stoclet J. Flavonoid compounds are potent inhibitor of cyclic AMP phosphodiesterase. Experientia 1978; 34: 1054–1055
- Chang W S, Chang Y H, Lu F J, Chiang H C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res 1994; 14: 501–506
- Da Silva E L, Tsushida T, Terao J. Inhibition of mammalian 15-lipoxygenase-dependent lipid peroxidation in low density lipoprotein by quercetin and quercetin monoglucosides. Arch Biochem Biophys 1998; 349: 313–320
- De Groot H, Hass W. Self-catalysed, O2-independent inactivation of NADPH-or dithionite-reduced microsomal cytochrome P-450 by carbon tetrachloride. Biochem Pharmacol 1981; 30: 2343–2347
- Demo A, Petrakis C, Kefalas P, Boskou D. Nutrient antioxidants in some herbs and Mediterranean plant leaves. Food Res Int 1998; 31: 351–354
- Deng Z, Tao B, Li X, He J, Chen Y. Effect of green tea and black tea on the blood glucose, the blood triglycerides, and antioxidation in aged rats. J Agr Food Chem 1998; 46: 3875–3878
- Duh P D, Tu Y Y, Yen G C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium. Ramat). Lebensm Wiss U Technol 1999; 32: 269–277
- Elliot A J, Scheiber S A, Thomas C, Pardini R S. Inhibition of glutathione reductase by flavonoids. A structure-activity study. Biochem Pharmacol 1992; 274: 1603–1608
- Erlund I, Alfthan G, Mäenpää J, Aro A. Tea and coronary heart disease: The flavonoid quercetin is more bioavailable from rutin in women than in men. Arch Intern Med 2001; 161: 1919–1920
- Ferriola P C, Cody V, Middleton E, Jr. Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem Pharmacol 1989; 38: 1617–1624
- Fewtrell C M, Gomperts B D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cell. Nature 1977; 265: 635–636
- Geleijnse J M, Launer L J, Hofman A, Pols H A, Witteman J C. Tea flavonoids may protect against atherosclerosis: The Rotterdam Study. Arch Intern Med 1999; 159: 2170–2174
- Geleijnse J M, Witteman J C, Launer L J, Lamberts S W, Pols H A. Tea and coronary heart disease: Protection through estrogen like activity?. Arch Intern Med 2000; 160: 3328–3329
- Gosselin R E, Smith R P, Hodge H C. Clinical toxicology of commercial product, 5th edition. William and Wilkins, Baltimore 1984; 101–107
- Grice H C. Safety evaluation of butylated hydroxytoluene (BHT) in the liver, lung and gastrointestinal tract. Food Chem Toxicol 1986; 24: 1127–1130
- Grice H C. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol 1988; 26: 717–723
- Hatano T, Edamatsu R, Mori A, Fujita Y, Yasuhara E. Effect of interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chem Pharm Bull 1989; 37: 2016–2021
- Hertog M G, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic B S, Toshima H, Feskens E JM, Hollman P CH, Katan M B. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 1995; 155: 381–386
- Keli S O, Hertog M G, Feskens E J, Krombout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen Study. Arch Intern Med 1996; 156: 637–642
- Lieber C S. Biochemical and molecular basis of alcohol induced injury to liver and other tissue. New Engl J Med 1988; 319: 1639–1650
- Lin C C, Tsai C C, Yen M H. The evaluation of hepatoprotective effects of Taiwan folk medicine ‘Teng-Khia-U’. J Ethnopharmacol 1995; 45: 113–123
- Lin C C, Yen M H, Chiu H F. The pharmacological and pathological studies on Taiwan Folk-Medicine (VI): The effects of Elephantopus scaber subsp. oblanceolata, E mollis Pseudoelephantopus spicatu. s. Am J Chinese Med 1991; 19: 41–50
- Lindhal M, Tagesson C. Selective inhibition of group II phospholipase A2 by quercetin. Inflammation 1993; 17: 573–582
- Liu H P. Catalysts of lipid peroxidation in meats.1: Linoleate peroxidation catalyzed by MetMb or Fe (ii)-EDTA. J Food Sci 1970; 35: 590–592
- Meir S, Kanner J, Akiri B, Hadas S P. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agr Food Chem 1995; 43: 1813–1817
- Mitra S K, Seshadri S J, Venkataranganna M V, Gopumadhavan S, Udupa V U, Sarma D N. Effect of HD-03-a herbal formulation in galactosamine-induced hepatopathy in rats. Ind J Physiol Pharmacol 2000; 44: 82–86
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004; 26: 211–219
- Murthy K NC, Jayaprakasha G K, Singh R P. Studies on antioxidant activity of pomegranate (Punica granatum.) peel extract using in vivo models. J Agr Food Chem 2002; 50: 4791–4795
- Oktay M, Culcin, Küfreviolu Í. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. Lebensm Wiss U Technol 2003; 36: 263–271
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr 1986; 44: 307–314
- Pasquali-Ronchetti, Bini A, Botti B, De Alojsio G, Fornieri C, Vannini V. Ultrastructural and biochemical changes induced by progressive lipid peroxidation on isolated microsomes and rat liver endoplasmic reticulum. Lab Invest 1980; 42: 457–468
- Rao G MM, Rao C V, Pushpangadan P, Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia. Linn. J Ethnopharmacol 2006; 103: 484–490
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio Med 1999; 26: 1231–1237
- Rice-Evans C A, Miller N J, Bolwell P G, Bramley P M, Pridham J B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res 1995; 22: 375–383
- Rosenblat M, Belinky P, Vaya J, Levy R, Hayek T, Coleman R, Merchav S, Aviram M. Macrophage encrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell mediated oxidation of low density lipoprotein. J Biol Chem 1999; 274: 13790–13799
- Rosen G M, Raukman E J. Spin trapping of superoxide and hydroxyl radicals. Method Enzymol 1984; 105: 198–209
- Sanchez-Moreno C, Larrauri J A, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int 1999; 32: 407–412
- Shen E S, Garry V F, Anders M W. Effect of hypoxia and carbon tetrachloride hepatotoxicity. Biochem Pharmacol 1982; 31: 3783–3793
- Slinkard K, Singleton V L. Total phenol analysis: automation and comparison with manual methods. American Journal of Enology and Viticulture 1977; 28: 49–55
- Tsai C C, Lin C C. Anti-inflammatory effects of Taiwan folk medicine ‘Teng-Khia-U’ on carrageenan-and adjuvant-induced paw edema in rats. J Ethnopharmacol 1999; 64: 85–89
- Yam M F, Basir R, Asmawi M Z, Ismail Z. Antioxidant and hepatoprotective effects of Orthosiphon stamineus. Benth standardized extract. Am J Chin Med 2007; 35: 117–128
- Yang J H, Lin H C, Mau J L. Antioxidant properties of several commercial mushrooms. Food Chem 2002; 77: 229–235
- Yang Y C, Lu F H, Wu J S, Wu C H, Chang C J. The protective effect of habitual tea consumption on hypertension. Arch Intern Med 2004; 164: 1534–1540
- Yen G C, Duh P D, Tsai C L. Relationship between antioxidant activity and maturity of peanut hulls. J Agr Food Chem 1993; 41: 67–70
- Yoshikawa M, Ninomiya K, Shimoda H, Nishida N, Matsuda H. Hepatoprotective and antioxidative properties of Salacia reticulata.: Preventive effects of phenolic constituents on CCl4-induced liver injury in mice. Biol Pharm Bull 2002; 25: 72–76