Abstract
The antimicrobial activities of chloroform, acetone, ethanol, and water extracts of 25 plants, mostly used as remedies against various diseases in Turkish traditional medicine, were tested against 10 pathogenic bacteria and one fungus (Candida albicans.) using the disk diffusion method. Among the tested plant species, Ziziphora clinopodioides. Lam (Labiatae), Thymus fallax. Fisch et Mey. (Labiatae), and three Hypericum. species [H. heterophyllum. Vent., H. hyssopifolium. Chaix. subsp. elongatum.(Ledeb.) Woron var. elongatum., and H. scabrum. L.] (Guttiferae) showed antimicrobial activity at a broader spectrum. In particular, chloroform, acetone, and ethanol extracts of Z. clinopodioides. inhibited the growth of all microbial species. Minimal inhibition concentration values of Z. clinopodioides. extracts were also found to be low. The antioxidant activity of the acetone, ethanol, and water extracts of 20 plants was also evaluated by lipid peroxidation inhibition and DPPH free radical scavenging methods. It was found that water, ethanol, and acetone extracts of Z. clinopodioides., T. fallax., three Hypericum. species, Artemisia santonicum. L. (Compositae), and Echinophora tenuifolia. L. subsp. sibthorpiena. (Umbelliferae) have strong antioxidant activities among the tested plant species. In general, there is a correlation between the antioxidant potential and total phenolic contents of the extracts. In light of the current study, it can be concluded that Z. clinopodioides. and T. fallax. may have potential use in the food industry as antioxidants and antimicrobial herbs, as well as pharmaceutical interest.
Introduction
In recent years, multiple-drug resistance in human pathogenic microorganisms has developed as a result of indiscriminate use of commercial antimicrobial drugs commonly used in the treatment of infectious diseases (Service, Citation1995). In addition, antibiotics are sometimes associated with undesirable adverse effects, including hypersensitivity, allergic reaction, and immune suppression (Davies, Citation1994; Enne et al., Citation2001). Many kinds of diseases have been treated with herbal remedies since ancient times. Herbal remedies are still being used extensively in many countries. Therefore, research into biologically active extracts and compounds from natural sources has been of great interest to scientists in an attempt to discover new sources for drugs that may be useful in combating infectious diseases. In recent years, a large number of studies dealing with antimicrobial screening of the extracts of medicinal plants have been reported (Sokmen et al., Citation1999; Keles et al., Citation2001; Erdogrul, Citation2002; Mukherjee et al., Citation2002; Rabanal et al., Citation2002; Dall'Agnol et al., Citation2003; Dulger & Gonuz, Citation2005; Fenner et al., 2005; Ozturk & Ercisli, Citation2005; Salehi et al., Citation2005). Turkish native herbs from both natural and cultivated sources are being more widely used on a commercial scale in the food industry, in traditional medicine, and for their flavoring properties (Baytop, Citation1999). For example, many Thymus. (Labiatae) and Ziziphora. (Labiatae) species are widely used as spices and in the treatment of infectious diseases (Baytop, Citation1999; Ismaili et al., Citation2004; Sokmen et al., Citation2004). Hypericum. (Guttiferae) species are commonly used in the treatment of skin wounds in traditional folk medicine (Baytop, Citation1999).
Lipid peroxidation is one of the major factors causing deterioration of foods during the storage and processing. Also, oxidized polyunsaturated fatty acids may induce aging and carcinogenesis (Halliwell & Gutteridge, Citation1989). Although there are some synthetic antioxidant compounds, such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), which are commonly used in processed foods, it has been reported that these compounds can have some side effects (Ito et al., Citation1983). Therefore, many researchers have focused their attention on natural antioxidants. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced in cells by different means (Davies, Citation1994; Halliwell, Citation1997). Exogenous sources of free radicals include tobacco smoke, ionizing radiation, certain pollutants, organic solvents, and pesticides (Halliwell & Gutteridge, Citation1989). ROS and RNS may cause DNA damage that could lead to mutation. All aerobic organisms, including humans, have antioxidant defenses that protect against oxidative damage (Davies, Citation2000; Sun et al., Citation1998). However, these natural antioxidant mechanisms can be inefficient, and hence dietary intake of antioxidant compounds becomes important (Espin et al., Citation2000). Natural antioxidants constitute broad ranges of compounds including phenolics, carotenoids, α-tocopherols, and vitamin C (Velioglu et al., Citation1998; Cakir et al., Citation2003, Citation2006). Medicinal plants and herbs are diverse sources of natural antioxidants. Therefore, there is a great interest concerning the antioxidant properties of medicinal plants and herbs (Velioglu et al., Citation1998; Cakir et al., Citation2003, Citation2006; Ismaili et al., Citation2004; Sokmen et al., Citation2004; Salehi et al., Citation2005; Erdemoglu et al., Citation2006; Ozgen et al., Citation2006).
This study was undertaken to determine the potential antimicrobial, antioxidant, and free radical scavenging activities of the extracts of some Turkish native herbs.
Materials and Methods
Plant material
The aerial parts of Hypericum heterophyllum. Vent. (Guttiferae), Echinophora tenuifolia. L. subsp. sibthorpiane. (Guss) (Umbelliferae), and Stachys recta. L. var. grandiflora. (Labiatae) were collected in the Gaziantep region of Turkey (southeastern Anatolia) in July 2005 at the flowering stages, and Salvia hydrangea. DC. ex Benth. (Labiatae) was collected in the Iğdir region of Turkey (northeastern Anatolia) in July 2005 at the flowering stages. Other plants used in the current study were collected in the Erzurum region of Turkey (eastern Anatolia) in July 2005 at the flowering stages. The plant samples were dried in shade and identified by Dr. Sengul. Voucher specimens of plant samples have been deposited at the herbarium of Atatürk University (Erzurum, Turkey) ().
Table 1 Plant species from Turkish flora screened for antimicrobial and antioxidant activities.
Extraction procedures
The aerial parts of the plant samples (50 g) were separately extracted with 150 mL chloroform (CHCl3), acetone, and ethanol (EtOH) at room temperature for 3-times. The organic solvents were evaporated to dryness under vacuum at low temperature using a rotary evaporator. To obtain the water extracts, 50 g plant sample was kept in 250 mL boiling water for 10 min and subsequently filtered. Then water solutions were lyophilized using a Labconco 117 freeze-dryer (Labconco Corp, Kansas City, MO, USA).
Antibacterial activity
Antibacterial activity assays of the extracts and commercial antibiotics were carried out by a disk diffusion method on nutrient agar (NA) medium for bacteria and potato dextrose agar (PDA) medium for fungi. Bacterial suspensions (108 CFU/mL final cell concentrations) were poured into Petri dishes (9 cm) from flasks containing 25 mL sterile NA and PDA and then were spread out by a sterile swab. The extracts solutions (10 mg/mL concentration) were prepared by dissolving in suitable organic solvents and the solutions sterilized by filtering through 0.45 μ m Millipore filters. Sterilized disks (6 mm) were impregnated with 100 μ L of the extract solutions and then were placed in the middle of the inoculated agar plates. Ofloxacin (OFX; 10 μ g/disk), sulbactam (30 μ g/disk) + cefoperazone (75 μ g/disk) (SCF; 105 μ g/disk), and netilmicin (NET; 30 μ g/disk) were used as positive controls. All of the reagents used in antibacterial activity assays and disks were obtained from Oxoid (Basingstoke, UK). Bacterial cultures were incubated for 24 h at 37 ± 2°C, whereas fungal cultures were incubated at 35 ± 2°C for 48 h. At the end of these periods, inhibition zones were measured in diameter (mm) around the disks. All of the tests were done in triplicate.
The minimal inhibition concentration (MIC) values were determined by using the modified microwell dilution method (Okeke et al., Citation2001): 100 μ L of suspension containing 108 CFU/mL of bacteria was spread on nutrient broth (NB). In the microwell dilution method, a twofold serial dilution of the extracts was prepared by diluting with dimethylsulfoxide (10%) to achieve a decreasing concentration range of 250 to 31.25 μ g/mL in the microplate. All test plates were incubated at 37°C for 24 h. The least concentration of each extract showing a clear zone of inhibition was taken as the MIC. Each assay in this experiment was repeated 3-times.
Antioxidant activity
Antioxidant activities of the acetone, ethanol, and water extracts of 20 plant species were determined using the thiocyanate method (Cakir et al., Citation2003, Citation2006). Briefly, extract solutions (30 μ g/mL) were mixed with 2.5 mL 0.02 M linoleic acid (Fluka emulsion containing equal weight of Tween-20 in pH 7.4 phosphate-buffered saline; Sigma), and the final volume was adjusted to 5 mL with phosphate-buffered saline (pH 7.4) in a test tube and incubated in darkness at 40°C. The amount of peroxide formed was determined by measuring absorbance at 500 nm after mixing of 0.1 mL sample with 0.1 mL FeCl2 (0.02 M) and 0.1 mL thiocyanate (30%) in 4.7 mL ethanol at intervals during incubation (Yen & Chen, 1995; Cakir et al., Citation2003, Citation2006). All measurements were done in triplicate.
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity
DPPH radical scavenging activities of acetone, ethanol, and water extracts of 20 plant species were measured as previously reported (Cakir et al., Citation2003, Citation2006). Briefly, a 1 mM solution of DPPH (Fluka) radical solution in methanol was prepared, and then 1 mL of this solution was mixed with 3 mL of sample solutions in ethanol. Final concentrations of the extracts were 50 and 100 μ g/mL. BHT was used as a positive control at 100 μ g/mL concentration. After 30 min incubation in the dark, the absorbance was measured at 517 nm. The assays were carried out in triplicate. A decrease in the DPPH solution absorbance indicates an increased DPPH radical scavenging activity. This activity is given as% DPPH radical scavenging that is calculated by the equation:
The DPPH solution without extract solution was used as control.
Amount of total phenolic compounds
Antioxidant compounds generally contain phenolic group(s). Because of this, amounts of phenolic compounds in each of the extracts were determined as described previously (Singleton et al., 1999). Briefly, extract solutions were adjusted to 4 mL by addition of distilled water and transferred to 10 mL Erlenmeyer flasks. Afterward, 0.25 mL Folin-Ciocalteu reagent (FCR; Fluka) was added to these mixtures, and after 3 min, 0.75 mL Na2CO3 solution was added. The final extract concentration was 100 μ g/mL. Subsequently, the mixture was shaken on a shaker for 2 h at room temperature, and then absorbance was measured at 760 nm. The assays were carried out in triplicate.
The same procedure was applied for gallic acid (Sigma) at a range of concentrations, and Abs760–gallic acid concentration graphic was obtained. Using the graphic, data of the extracts are given as gallic acid equivalents.
Statistical analyses
In the measurements of all activities, three individual measurements were made, and results were calculated as means of these measurements. Statistical calculations were carried out using SPSS 9.0 software. To determine whether there were significant differences between activities of samples analysis of variance (ANOVA) was applied. Values of p < 0.05 were considered significantly different (α = 0.05).
Results and Discussion
Antimicrobial activities of extracts
In the current study, the antimicrobial activities of chloroform, acetone, ethanol, and water extracts of 25 plant species () were studied using the disk diffusion method. These plants are widely used as folk remedies against various diseases in Turkey. The extract yields varied from 0.25% to 28.30% (w/w) (). As shown in , the plant species exhibited the antimicrobial activity producing inhibition zone diameters varying from 8.0 to 35.0 mm, depending on susceptibility of the tested bacteria. P. vulgaris. and B. megaterium. were the most sensitive bacteria to the plant extracts. The MICs of the extracts showing inhibition zones more than 10 mm were also determined ().
Table 2 Yields of the extracts.
Table 3 Antimicrobial activity of the plants as mean of inhibition diameter zone (mm); MIC in μ g/mL is given in parentheses.
Among the tested plant species, chloroform, ethanol, and acetone extracts of Ziziphora clinopodioides. strongly inhibited the growth of all bacterial species, with the exception of the ethanol extract of this species against B. megaterium.. Several MIC values were also found to be low (). Nevertheless, the water extract of Z. clinopodioides. was effective against only S. aureus.. The water extracts of the plant species were, in general, not as effective against the microbial species as organic solvent extracts. The other plant species that exhibited significant antimicrobial activity over a relatively broad spectrum were Thymus fallax., three Hypericum. species (H. scabrum., H. hyssopifolium. subsp. elongatum. var. elongatum., H. heterophyllum.), and two Artemisia. (Compositae) species (A. santonicum. and A. spicigera.). The largest inhibition zones (35 mm) and lowest MIC values were observed for the chloroform and acetone extracts of Z. clinopodioides. against P. vulgaris.. As can be seen in , E. coli. and C. albicans. were the most resistant microbial species against the extracts. Among the plant species tested, only Z. clinopodioides., A. santonicum., and A. spicigera. showed anticandidal activity.
The current results show that Z. clinopodioides. had a remarkable antibacterial and anticandidal activity among the tested plants. These results are compatible with the results reported by Salehi et al. (Citation2005), who reported that a methanol extract of this plant exhibited antibacterial activity against B. subtilis., S. aureus., and E. coli.. However, acetone extract of Iranian Z. clinopodioides. subsp. rigida. has not shown any antibacterial activity (Salehi et al., Citation2005). Convensely, in the current study, the acetone extract of Turkish Z. clinopodioides. showed a remarkable antibacterial activity. These differences may be attributed to the genotypic variation and/or climatic conditions.
The acetone extract of T. fallax. inhibited the growth of nine bacterial species (). Likewise, recent studies showed that Thymus. species growing in Turkish flora have strong antibacterial activity over a broad spectrum (Vardar-Unlu et al., Citation2003; Ozturk & Ercisli, Citation2005).
Previous reports also showed that some Hypericum. species growing in various regions of the world have a remarkably broad spectrum of antimicrobial activities (Herrera et al., Citation1996; Sokmen et al., Citation1999; Mukherjee et al., Citation2002; Rabanal et al., Citation2002; Dall'Agnol et al., Citation2003; Dulger et al., Citation2005; Dulger & Gonuz, Citation2005; Fenner et al., 2005). However, none of those studies were about the Hypericum. species that were the subjects of the current study. The three Hypericum. species tested in the current study showed remarkable antibacterial activity (). In contrast with antibacterial activity, these Hypericum. species had no anticandidal activity against C. albicans. (). Similarly, Fenner et al. (2005) have found that the extracts of some Hypericum. species did not show any activity against C. albicans.. Other reports on Hypericum. showed that this genus contains phloroglucinols, flavonoids, tannins, saponins, benzopyrans, and xanthones (Ishiguro et al., Citation1994; Yamaki et al., Citation1994; Cakir et al., Citation2003; Dall'Agnol et al., Citation2003, Citation2005). It has also been demonstrated that phloroglucinol derivatives isolated from some Hypericum. species have antibacterial activity (Ishiguro et al., Citation1994; Yamaki et al., Citation1994; Dall'Agnol et al., Citation2005). Therefore, the antibacterial activities of Hypericum. species determined in the current study may be attributed to their phloroglucinol derivatives.
Antioxidant and DPPH radical scavenging activities of the extracts
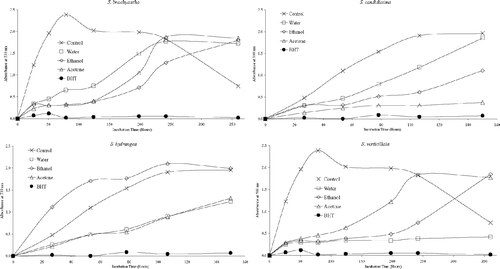
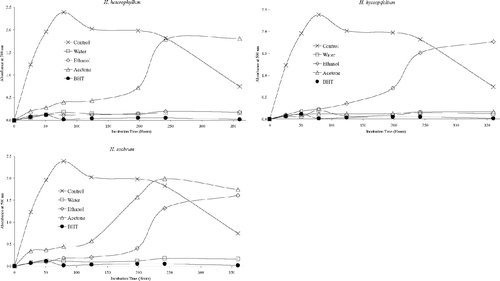
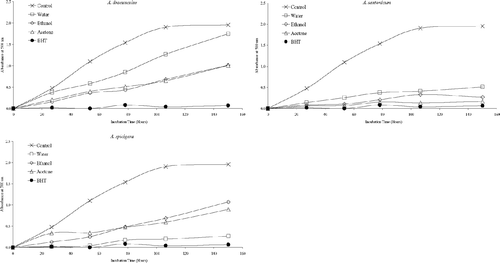
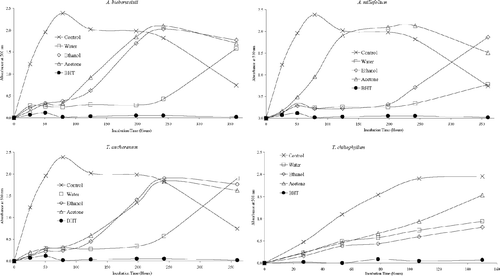
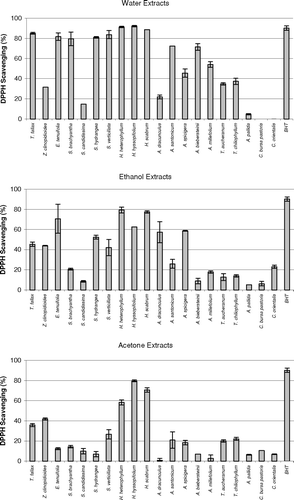
Antioxidant activities of acetone, ethanol, and water extracts of 20 plant species were determined using the thiocyanate method. Chloroform extracts of the plant samples were not tested for antioxidant activity due to solubility problems in the emulsion system. As can be seen from , , , , , , the extracts isolated from plant samples showed various degrees of inhibition of the oxidation of linoleic acid compared with the control. It can be concluded from the figures that Z. clinopodioides., T. fallax., three Hypericum. species, A. santonicum., and Echinophora tenuifolia. have strong antioxidant activities among the tested plant species. For instance, ethanol and water extracts of Z. clinopodioides. and H. heterophyllum., water and acetone extracts of T. fallax., and acetone and ethanol extracts of H. hyssopifolium. and E. tenuifolia. strongly inhibited the oxidation of linoleic acid. However, the extracts of the other tested plant species in the current study have moderate, weak, or no antioxidant activities (, , ).
Figure 1 Inhibition of lipid peroxidation by the extracts of T. fallax, Z. clinopodioides., and E. tenuifolia..
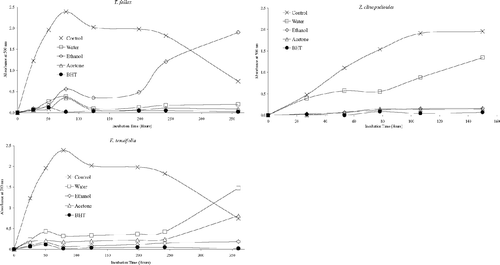
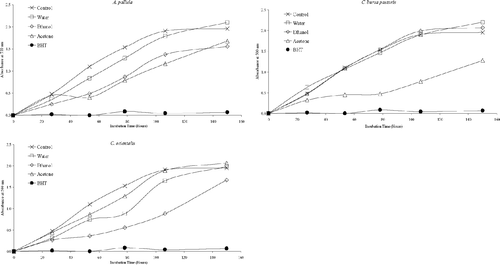
Figure 6 Inhibition of lipid peroxidation by the extracts of A. pallida., C. bursa pastoris., and C. orientalis. species.
The free radical scavenging activities of the extracts of 20 plant samples were determined using a stable DPPH free radical. The results are shown in . The figure shows that water was found to be the best solvent for extracting the DPPH radical scavenging components from the plant samples compared with ethanol and acetone. Hence, it can be suggested that polar compounds present in the herb are mainly responsible for its free radical scavenging activity. It seems that results of DPPH radical scavenging activities of the plant samples do not always correlate with their antioxidant activities. For instance, as shown in , all extracts of Hypericum. species strongly inhibited the oxidation of the linoleic acid and also showed potent DPPH radical scavenging activities. However, Z. clinopodioides. and T. fallax. extracts were superior to those of the other plants for inhibiting linoleic acid oxidation, whereas these species showed relatively low DPPH radical scavenging activities. Nevertheless, all extracts of Crambe orientalis. L. var.orientalis. (Cruciferae), Capsella bursa-pastoris. (Cruciferae), and Salvia candidissima. Vahl. subsp. candidissima.(Labiatae) showed weak DPPH radical scavenging activities as well as weak inhibition of linoleic acid oxidation.
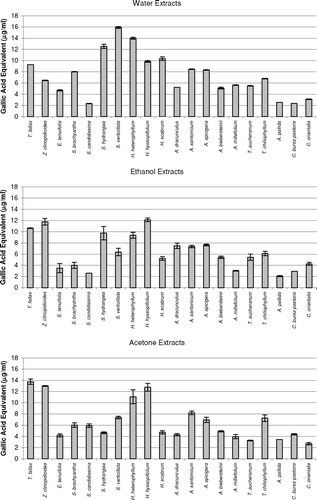
It is believed that the phenolic and/or polyphenolic compounds biosynthesized in the plant sample might be responsible for antioxidant activity (Cakir et al., Citation2003, Citation2006). Hence, total phenolic compound contents of the extracts of 20 plant species were determined. The amounts of total phenolic compounds present in the extracts are shown in as gallic acid equivalent. As shown in this figure, in general, the extracts of three Hypericum. species, T. fallax., Z. clinopodioides., and S. hydrangea. contained relatively high content of phenolic compounds. These results would suggest that there is a correlation between antioxidant potential and total phenolic content of the extracts. This correlation was confirmed by ANOVA treatment (α = 0.05). Likewise, the plant samples such as S. candidissima., Alcea pallida. Waldst and Kit (Malvacea), and C. bursa-pastoris. showed relatively weak antioxidant and free radical scavenging activities and also contain relatively low amounts of phenolic compounds. However, all extracts of E. tenuifolia. showed potent inhibitory effect on the peroxidation of linoleic acid and free radical scavenging activity, whereas their phenolic contents were found to be relatively low. Therefore, the antioxidant potential of E. tenuifolia. can be attributed to its nonphenolic components. It is interesting to find that the ethanol extract of S. hydrangea. contains a relatively high content of phenolic compounds but significantly increased the peroxidation of linoleic acid ( and ).
The current results show that Z. clinopodioides., T. fallax., and three Hypericum. species (H. scabrum., H. hyssopifolium., and H. heterophyllum.) have strong antioxidant potential. This may be due to their high phenolic contents. Thymus. as well as Ziziphora. species are of botanical and pharmaceutical interest due to their characteristic scent or taste (Baytop, Citation1999; Ismaili et al., Citation2004; Sokmen et al., Citation2004). Various Thymus. species are aromatic plants of Mediterranean flora, commonly used as spices, food preservatives, and traditional medicine (Baytop, Citation1999; Ismaili et al., Citation2004; Sokmen et al., Citation2004). Consequently, there are numerous studies on the antioxidant potential of Thymus. species growing in various regions of the world (Ismaili et al., Citation2004; Sokmen et al., Citation2004; Agbor et al., Citation2005; Ozgen et al., Citation2006; Vitalini et al., Citation2006). Recent reports show that various Thymus. and several Ziziphora. species have antioxidant potential (Ismaili et al., Citation2004; Sokmen et al., Citation2004; Agbor et al., Citation2005; Salehi et al., Citation2005; Ghafari et al., Citation2006; Ozgen et al., Citation2006; Vitalini et al., Citation2006). As shown in and , all extracts of Z. clinopodioides. and T. fallax. have potent antioxidant and free radical scavenging activities.
Reports have demonstrated that Hypericum. species contain many phenolic compounds such as flavonoids, phoroglucinols, bisanthraquinones, and xanthones, most of which have antioxidant activity (Zheng & Wang, Citation2001; Couladis et al., Citation2002; Cakir et al., Citation2003; Skerget et al., Citation2005). Thus, the strong antioxidant potential of Hypericum. species tested in the current study may be attributed to their phenolic metabolites.
Recently, Tepe et al. (Citation2006) studied the antioxidant activities of methanol extracts of six Salvia. (S. caespitosa., S. hypargeia., S. euphratica., S. sclarea., S. candidissima., and S. aethiopis.) species and found that S. candidissima. has higher antioxidant activity than the rest. As shown in , acetone and ethanol extracts of this species exhibit significant antioxidant activity. Similar results for antioxidant activities of Salvia. species have been found by others (Malencic et al., Citation2000; Yildirim et al., Citation2000; Couladis et al., Citation2002). Furthermore, two potent antioxidant phenolic compounds from S. plebei. were also reported (Weng & Wang, 2000; Gu & Weng, Citation2001).
In conclusion, Thymus. and Ziziphora. species have botanical and pharmaceutical interest due to their characteristic scent or taste, and various Thymus. species are aromatic plants of Mediterranean flora, commonly used as spices, food preservatives, and traditional medicine. The current results show that the aerial parts of Z. clinopodioides. and T. fallax. have both broad-spectrum antimicrobial activities and potent antioxidant and free radical scavenging activities. From these results, it can be concluded that Z. clinopodioides. and T. fallax. may find new benefits as natural sources in the food and pharmaceutical industries due to their strong antimicrobial and antioxidant activities. The components responsible for their antimicrobial and antioxidant activities have not been identified. This will be the aim of further studies.
References
- Agbor G A, Oben J E, Ngogang J Y, Cai X X, Vinson J A. Antioxidant capacity of some herbs/spices from Cameroon: A comparative study of two methods. J Agric Food Chem 2005; 53: 6819–6824
- Baytop T. Therapy with Medicinal Plants in Turkey; Today and in Future. Istanbul University Press, Istanbul 1999; 166–167
- Cakir A, Duru M E, Harmandar M, Izumi S, Hirata T. The volatile constituents of Stachys recta. L. and Stachys balansae. L. from Turkey. Flavour Fragr J 1997; 12: 215–218
- Cakir A, Mavi A, Yildirim A, Duru M E, Harmandar M, Kazaz C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium. L. by activity-guided fractionation. J Ethnopharmacol 2003; 87: 73–83
- Cakir A, Mavi A, Kazaz C, Yildirim A, Kufrevioglu O I. Antioxidant activities of the extracts and components of Teucrium orientale. L. var. orientale.. Turk J Chem 2006; 30: 483–494
- Couladis M, Tzakou O, Verykokidou E, Harvala C. Screening of some Greek aromatic plants for antioxidant activity. Phytother Res 2002; 17: 194–195
- Dall'Agnol R, Ferraz A, Bernardi A P, Albring D, Nor C, Sarmento L, Lamb L, Hass M, von Poser G, Schapoval E ES. Antimicrobial activity of some Hypericum. species. Phytomedicine 2003; 10: 511–516
- Dall'Agnol R, Ferraz A, Bernardi A P, Albring D, Nor C, Schapoval E ES, von Poser G L. Bioassay-guided isolation of antimicrobial benzopyrans and phloroglucinol derivatives from Hypericum. species. Phytother Res 2005; 19: 291–293
- Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science 1994; 264: 375–382
- Davies K JA. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000; 50: 279–289
- Dulger B, Gonuz A, Bilen S. Antimicrobial studies on three Hypericum. species from Turkey. S Afr J Bot 2005; 71: 100–103
- Dulger B, Gonuz A. Antibacterial activity of the endemic Hypericum kazdaghensis.. Fitoterapia 2005; 76: 237–239
- Enne V I, Livermore D M, Stephens P, Hall L MC. Peristence of sulphanamide resistance in Escherichia coli. in the UK despite national prescribing restriction. Lancet 2001; 28: 1325–1328
- Erdemoglu N, Turan N N, Cakici I, Sener B, Aydin A. Antioxidant activities of some Lamiaceae plant extracts. Phytother Res 2006; 20: 9–13
- Erdogrul O T. Antibacterial activities of some plant extracts used in folk medicine. Pharm Biol 2002; 40: 269–273
- Espin J C, Soler-Rivas C, Wichers H J. Characterization of the total free radical scavenger capacity of vegetable oils and oil fractions using 2,2-diphellyl-1-picrylhydrazyl radical. J Agric Food Chem 2000; 48: 648–656
- Feneer R, Sortino M, Kuze Rates S M, Dall'Agnol R, Ferraz A, Bernardi A P, Albring D, Nor C, von Poser G, Schapoval E, Zacchino S. Antifungal activity of some Hypericum. species. Phytomedicine 2005; 12: 236–240
- Ghafari H, Yasa N, Mohammadirad A, Dehghan G, Zamani M J, Nikfar S, Khorasani R, Minaie B, Abdollahi M. Protection by Ziziphora clinopoides. of acetic acid-induced toxic bowel inflammation through reduction of cellular lipid peroxidation and myeloperoxidase activity. Hum Exp Toxicol 2006; 25: 325–332
- Gu L W, Weng X C. Antioxidant activity and components of Salvia plebeia. R.Br.—A Chinese herb. Food Chem 2001; 73: 299–305
- Halliwell B. Antioxidants and human disease: A general introduction. Nutr Rev 1997; 55: S44–S49
- Halliwell B, Gutteridge J M. Free Radicals in Biology and Medicine. Clarendon Press, Oxford 1989; 23–30
- Herrera R M, Perez M, Mertin Herrera D A, Lopez Garcia R, Rabanal R M, Arias A. Antimicrobial activity of extracts from plants endemic to the Canary Islands. Phytother Res 1996; 10: 364–366
- Ishiguro K, Nagata S, Fukumoto H, Yamaki M, Isoi K. An isopentylated flavonol from Hypericum japonicum.; Phloroglucinol derivatives from Hypericum japonicum.. Phytochemistry 1994; 35: 469–471
- Ismaili H, Milella L, Fkih-Tetouani S, Ilidrissi A, Camporese A, Sosa S, Altinier G, Della Loggia R, Aquino R. In vivo. topical anti-inflammatory and in vitro. antioxidant activities of two extracts of Thymus satureioides. leaves. J Ethnopharmacol 2004; 91: 31–36
- Ito N, Fukushima S, Hassegawa A, Shibata M, Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst 1983; 70: 343–347
- Keles O, Ak S, Bakirel T, Alpinar K. Screening of some Turkish plants for antibacterial activity. Turk J Vet Anim Sci 2001; 25: 559–565
- Malencic D J, Gasic O, Popovic M, Boza P. Screening for antioxidant properties of Salvia reflexa. Hornem. Phytother Res 2000; 14: 546–548
- Mukherjee P K, Saritha G S, Suresh B. Antimicrobial potential of two different Hypericum. species available in India. Phytother Res 2002; 16: 692–695
- Okeke M I, Iroegbu C U, Eze E N, Okoli A S, Esimone C O. Evaluation of extracts of the root of Landolphia owerrience. for antibacterial activity. J Ethnopharmacol 2001; 78: 119–127
- Ozgen U, Mavi A, Terzi Z, Yildirim A, Coskun M, Houghton P J. Antioxidant properties of some medicinal Lamiaceae (Labiatae) species. Pharm Biol 2006; 44: 107–112
- Ozturk S, Ercisli S. Broad-spectrum antibacterial properties of Thymus fallax.. Pharm Biol 2005; 43: 609–613
- Rabanal R M, Arias A, Prado B, Hernandez-Perez M, Sanchez-Mateo C C. Antimicrobial studies on three species of Hypericum. from the Canary Islands. J Ethnopharmacol 2002; 81: 287–292
- Salehi P, Sonboli A, Eftekhar F, Nejad-Ebrahimi S, Yousefzadi M. Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides. subsp rigida. (Boiss.) RECH. f. from Iran. Biol Pharm Bull 2005; 28: 1892–1896
- Service R F. Antibiotics that resist resistance. Science 1995; 270: 724–727
- Singleton V L, Orthofer R, Lamuela-Raventos R M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1998; 299: 152–178
- Skerget M, Kotnik P, Hadolin M, Hras A R, Simonic M, Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem 2005; 89: 191–198
- Sokmen A, Jones B M, Erturk M. Antimicrobial activity of extracts from the cell cultures of some Turkish medicinal plants. Phytother Res 1999; 13: 355–357
- Sokmen A, Gulluce M, Akpulat H A, Daferera D, Tepe B, Polissiou M, Sokmen M, Sahin F. The in vitro. antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius.. Food Control 2004; 15: 627–634
- Sun J, Chen Y, Li M, Ge Z. Role of antioxidant enzymes on ionizing radiation resistance. Free Radic Biol Med 1998; 24: 586–593
- Tatli A. Important Range Plants of Erzurum Province. Food and Agricultural Organization of the United Nations Press, Ankara, Turkey 1988; 1–77
- Tepe B, Sokmen M, Akpulat H A, Sokmen A. Screening of the antioxidant potentials of six Salvia. species from Turkey. Food Chem 2006; 95: 200–204
- Vardar-Unlu G, Candan F, Sokmen A, Daferera D, Polissiou M, Somken M, Donmez E, Tepe B. Antibacterial and antioxidant activity of the essential oil and methanol extract of Thymus pectinatus Fisch. et. Mey var. pectinatus. (Lamiaceae). J Agric Food Chem 2003; 51: 63–67
- Velioglu Y S, Mazza G, Gao L, Oomah B D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 1998; 46: 4113–4117
- Vitalini S, Grande S, Visioli F, Agradi E, Fico G, Tome F. Antioxidant activity of wild plants collected in Valsesia, an Alpine region of Northern Italy. Phytother Res 2006; 20: 576–580
- Weng X C, Wang W. Antioxidant activity of compounds isolated from Salvia plebeia.. Food Chem 2001; 71: 489–493
- Yamaki M, Miwa M, Ishiguro K, Takagi S. Antimicrobial activity of naturally-occurring and synthetic phloroglucinols against Staphylococcus aureus.. Phytother Res 1994; 8: 112–114
- Yildirim A, Mavi A, Oktay M, Kara A, Algur O F, Bilaloglu V. Comparison of antioxidant and antimicrobial activities of tilia (Tilia Argentea Desf Ex. DC), sage (Salvia Triloba. L.), and black tea (Camellia Sinensis.) extracts. J Agric Food Chem 2000; 48: 5030–5034
- Zheng W, Wang S Y. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 2001; 49: 5165–5170