Abstract
The functional approach and molecular biological mechanism of polysaccharides from Dendrobium huoshanense. C. Z. Tang et S. J. Cheng (Orchidaceae) on anticataract activity were assessed. Rats with intraperitoneal injection of streptozotocin (STZ; 45 mg kg− 1) were used as the animal model of diabetic cataract. Administration of polysaccharides from Dendrobium huoshanense. (HPS) caused a significant body weight increase at dosages of 50, 100, and 200 mg kg− 1 day− 1, whereas blood sugar was decreased. Compared with rats without therapy, the opacity of lenses was abated in groups treated with HPS. Taking into account NO and NOS levels in lenses, 70.2 μ mol mg− 1 protein of NO and 23.4 U mg− 1 protein of NOS were obtained with a dosage of HPS at 200 mg kg− 1day− 1, which all decreased more than 33% compared with those in the rats without therapy. RT-PCR analysis also showed that the inhibitory response of iNOS gene expression was in a dose-dependent manner with HPS treatment. To restrain advanced glycation end products (AGE) synthesis was another phenomenon in this experiment, and the suppression ratio reached 40.2% at 200 mg kg− 1day− 1 treatment, compared with the rats without therapy. These results indicated that polysaccharide of D. huoshanense. is a new pharmaceutical agent for prevention and cure of diabetic cataract.
Introduction
Cataract, the opacification of the eye lens, is the leading cause of blindness worldwide. Among various factors leading to cataract, such as aging, nutritional inadequacy, metabolic and inherited defects, UV radiation and smoking, diabetes has been considered to be one of the major risk factors in Western countries (Ettl et al., Citation2004; Palla et al., Citation2005). It is estimated that half of the world's blind population were blinded because of diabetic cataract, with its onset is comparatively at a younger age in developing countries (Palla et al., Citation2005). As life expectancy in these countries increases, the number will be fast growing (Reidy et al., Citation2002). Cataract is thus a major health disease and a serious social problem. Cataract surgery is currently a major treatment method, but accompanied surgical complications can result in visually disabling cataract (Vats et al., Citation2004). Recently, there is considerable interest in identifying preventive factors that may delay the onset or progression of lens opacities, and herbs have been used to prevent or delay cataract (Rahman, Citation2003). Dendrobium huoshanense. C. Z. Tang et S. J. Cheng (Orchidaceae) is a famous herb in traditional Chinese medicine for eye protection (Zha et al., Citation2007). Polysaccharides from Dendrobium. species have been shown to be effective for decreasing blood sugar levels and scavenging free oxygen radicals (Lin et al., Citation2003). Because diabetic cataract development has been suggested to be related to hyperglycemia and oxidative mechanisms (Van et al., 1992; Zoric, Citation2003), we speculated that the polysaccharides from D. huoshanense. might have a significant inhibitory effect on diabetic cataract development. Such an ethnomedical approach would be practical, cost-effective, and logical for prevention/treatment of diabetic cataract. The aim of the current study was to investigate the effect of polysaccharides from D. huoshanense. on diabetic cataract development and its possible functional approach.
Materials and Methods
Reagents
Streptozotocin (STZ) was purchased from Sigma (St. Louis, MO, USA). TRIzol was from Gibco-BRL (Gaithersburg, MD, USA). M-MLV reverse trancriptase was from Promega Corporation (Madison, WI, USA). Proteinase K was from AMRESCO Inc. (Solon, OH, USA). Collagenase type IV was purchased from Invitrogen Corporation (Carlsbad, CA, USA). l-Hydroxyproline was from Solarbio Co. Ltd. (Beijing, China).
Preparation of polysaccharide of D. huoshanense.
D. huoshanense. was collected in the Dabieshan mountain of China in July 2001 and propagated under controlled conditions (Zha et al., Citation2006). Plant material was identified by Professor Runlong Lu from the University of Science and Technology of China (USTC). A voucher specimen is kept at the School of Life Sciences of USTC. Stems of D. huoshanense. were dried in a constant temperature oven and ground in an electric grinder into a fine powder through a 200-mesh. The powdered material (500 g) was pre-extracted for 48 h in a Soxhlet system with acetone and subsequently for another 48 h with MeOH. The extract was discarded. The residue was dried at 40°C and extracted 3 times with five volumes of hot distilled water and 2.5% quantity of polyvinylpyrrolidone. The combined extracts were concentrated and centrifuged at a speed of 12,000 × g. for 30 min. The supernatant was poured into a four-fold volume of 95% EtOH and centrifuged, and the precipitate was dissolved in distilled water. This process was repeated 3 times. The precipitate was then washed with Sevag reagent (isoamyl alcohol and CHCl3 in a 1:4 ratio) (Bao et al., Citation2002) and dried in a vacuum, to give the crude polysaccharide of D. huoshanense. (HPS) for all experiments. The content of HPS was determined by the phenol-sulfate acid method (Dubios, Citation1956).
Preparation of rat models
Sprague-Dawley rats (male and female) weighing approximately 180 g were obtained from the Animal Research Center of Anhui Medical University of China. Rats were housed together in a controlled environment of natural daylight cycle. After 2 days, the rats were used to induce diabetes. After overnight fasting, rats were injected with freshly prepared STZ at a single dose of 45 mg kg− 1 body weight in 0.1 M citrate buffer (pH 4.5). Three days after administration of STZ, blood sugar levels were determined. Rat blood sugar levels were measured by Roche Accu-Chek (Roche Diagnostics Ltd., Manheim Germany) Active and exhibiting blood sugar levels > 16 mmol L− 1 were included in the study.
Experimental design
All HPS was dissolved in distilled water and given orally once daily using a feeding tube. Animals were randomized in the following groups: NC group (normal control group, received only physiologic saline solution), DC group (diabetic group, made diabetic previously and received physiologic saline solution), and HPS-treated groups (groups I–III, made diabetic previously and received HPS solution, 50, 100, and 200 mg kg− 1day− 1, respectively). Animals were housed under normal laboratory conditions and allowed free access to feed and drinking water.
Slit-lamp examination of the cataract
Both eyes of rats were dilated using compound tropicamide eye drops before examination under a slit lamp (pocket ophthalmoscope YZIIC; 66 Vision Tech Co., Ltd. Suzhou city, China) on the 45th day after diabetic cataract rat treatment with HPS solution.
Preparation of lens
Rats were sacrificed by decapitation after feeding 45 days. The dissected lenses, after a brief rinse with physiological saline, were collected and stored at −80°C until processed for analyses of redox-related gene expression, enzymatic activity bioassay, and protein measurements.
Nitric oxide and nitric oxide synthase activity bioassay
The lenses were homogenized in physiologic saline solution on ice. A clear supernatant was obtained by centrifugation at 3000 × g. for 15 min and used for analysis. Nitric oxide (NO) and nitric oxide synthase (NOS) activity was measured by NO kits and NOS kits, respectively, according to the recommendation of the manufacturer (TaKaRa Biotechnology Co., Ltd., Dalian, China).
RT-PCR analysis of iNOS
The lens RNA in different dosage groups was extracted with TRIzol reagent according to the recommendation of the manufacturer. RNA concentration was determined by measuring the optical density at 260 nm.
All PCR primer pairs specific for iNOS and β-actin were synthesized by Shanghai Biotechnology Company (Shanghai, China). The primer sequences and expected size of the PCR products are shown in (Hao et al., Citation2005). β-Actin (420 bp) was used as standard in this study.
Table 1 Sequences of the primers used in RT-PCR.
RT-PCR was carried out according to Hao et al. (Citation2005) with some modifications. RNA was reverse-transcribed at 42°C for 1 h in a 20-μ L reaction mixture after heating at 70°C for 15 min. The reverse mixture was the same as that described by the RT-PCR kit and used following the manufacturer's instructions. RT samples were stored at −80°C until subjected to PCR amplification. PCR was performed on Multicolor Real time PCR Detection system (Applied Biosystems, Forester City, CA, USA). The following components were added to the RT sample to make up 25 μ L reaction mix: 0.5 μ L cDNA, 12.5 μ L iQ SYBR Green Supermix, 1 μ L each of 5′ and 3′ primer (10 pmol μ L− 1), and 10 μ L sterile water. After an initial denaturation at 94°C for 2 min, thermal conditions followed 35 cycles of 45 s of denaturation at 94°C, 45 s of annealing at 55°C, and 45 s of extension at 72°C, and extension with fluorescence monitoring at 70°C for 30 s, after which the reaction was stopped (96°C for 2 min), cooled (20.8°C for 1 min), and melted (65°C to 95°C with plate readings every 0.5°C).
The reaction products were visualized by electrophoresis in 1.2% agarose gel containing 0.5 μ g mL− 1 ethidium bromide. λ DNA/EcoRI + Hind III were run in parallel as the molecular weight marker.
Advanced glycation end products (AGEs) determination
The AGEs determination was carried out according to Zhao et al. (Citation2002) with some modifications. The lenses were added to 5 mL ice-cold phosphate buffer (pH 7.4) saline solution (PBS buffer), triturated to make homogeneous, and centrifuged at a speed of 4000 × g. for 15 min. The precipitate was washed with 2 mL carbinol followed by 5 mL distilled water, which was repeated 3 times. The precipitate was suspended in 2 mL medium (1 mg mL− 1 pepsin, 0.5 M acetic acid) and left for 18 h at 4°C, and subsequently centrifuged at a speed of 12,000 × g. for 30 min. The precipitate was washed 2 times with CaCl2 (0.1 M) in Tris-HCl buffer (pH 7.4). The supernatant was discarded, and the precipitate added into 2 mL medium (0.1 mg mL− 1 IV collagenase, 0.1 mg mL− 1 proteinase k, 1 μ L mL− 1 toluene, Tris-HCl buffer). After oscillating gently at 37°C for 72 h, the solution was centrifuged at 12,000 × g. for 30 min. The supernatant was determined by fluorescence spectrophotometer equipped with an excitation wavelength of 370 nm and 440 nm emission wavelength. The intensity of fluorescence was denoted with arbitrary fluorescence unit (AFU), and 1 mg mL− 1 fluorescence of hydroxyproline was designated as an AFU.
After determination of fluorescence, the supernatant was transferred to an equivalent volume of HCl (6 M) in a hermetic vitreous tube. The tube was placed in oil at 110°C for 24 h. The supernatant was subsequently adjusted to pH 6.0 with 6 M NaOH. The supernatant (2 mL) was allowed to react with 1 mL 0.05 M chloramine-T at 30°C for 20 min. The reaction was arrested by adding 1 mL acetaldehyde–perchloride acid medium at 60°C for 5 min. The product was detected at 550 nm until the temperature of the mixture was reduced to room temperature within 2 h. The content of hydroxyproline in collagen was 14% and stable, so the quantity of collagen was obtained by conversion.
Statistical analysis
All results are expressed as the mean ± SD. The data were analyzed statistically using one-way analysis of variance (ANOVA), and the data means were compared using Duncan's multiple range tests with the level of significance set at 5%.
Results
Body weight and blood sugar
The effects of STZ and HPS at different concentrations on body weight and blood glucose levels are summarized in . There was an increase in the food intake in all the diabetic groups (DC and I–III) compared with the normal control group (NC). Although the weights of all treated groups were lower than those of NC group, administration of HPS caused a significant weight increase of 10%, 15%, and 16% at concentrations of 50, 100, and 200 mg kg− 1day− 1, respectively, after 45 days of feeding. Taking into account blood sugar, the levels were markedly enhanced up to six-fold in the diabetic controls compared with normal controls (p < 0.05). The three concentrations of HPS all caused significant reduction in blood sugar levels (p < 0.05) in a dose-dependent manner. The lowest sugar concentration in blood (12.24 mmol L− 1) was observed in the groups treated with 200 mg kg− 1day− 1 HPS.
Table 2 Comparison of weight and blood sugar of rats after feeding for 45 days.
Observation of opacities
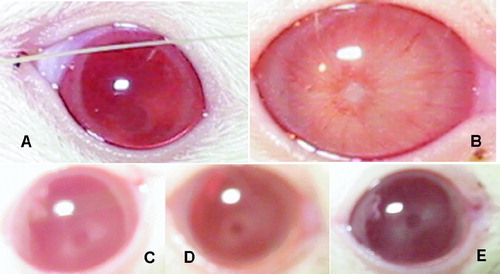
All the lenses in NC were clear, whereas those in DC had significant opacity. Results also showed that compared with DC, the opacity of lenses was mitigated in a dose-dependent manner in the groups treated with HPS ().
Figure 1 HPS retards the progression of diabetic cataract in vivo. in rats. (A) Normal rat eyes; (B) Generation of cataract in rat in vivo. by the administration of STZ without therapy; (C, D, E) the eyes of rats in 50 mg kg− 1day− 1, 100 mg kg− 1day− 1, and 200 mg kg− 1day− 1 HPS-treated groups, respectively.
Nitric oxide and nitric oxide synthase activity level
The NO level and NOS activity of normal group were 50.95 μ mol mg− 1 pro and 14.5 U mg− 1 protein, respectively, and were significantly lower than those in the STZ groups. Administration of HPS caused a significant decrease in NO level and NOS level, especially at 200 mg kg− 1day− 1 HPS treatment, which were all approximate two-fold the NO and NOS levels of control treated only with STZ, although still higher than the normal group at 45 days. The decreasing amount depended on the concentrations of HPS ().
Table 3 The comparison of the NO content and NOS activities of lens epithelium cells in different groups at 45 days.
RT-PCR detection of iNOS
Our relative quantification assay using β-actin as an external standard was configured for PCR amplification of both iNOS and β-actin. Quantification of mRNA was accomplished by analysis of fluorescence curves and determination of crossing threshold (the cycle at which the fluorescent signal rises above background, C.t) for each sample. Samples with a higher preamplification target concentration showed an earlier cycle threshold ( and ). So the lower C.t. implied the higher level of iNOS. This value was normalized to a β-actin transcript (opposite C.t) to adjust differences in the amount of mRNA present in the samples (). The iNOS gene had a high expression in the DC group by analysis of the opposite C.t. value. On the contrary, the expression of iNOS showed an inhibition with an increased opposite C.t of 0.75, 0.81, and 0.87 at 50, 100, and 200 mg kg− 1day− 1, respectively. The results were also verified in an electrophoresis experiment with 1.2% agarose gel (). Melting curve analysis was performed to measure the specificity of the PCR product (). The exclusive melting peak was presented at 87.8°C, which indicated that there were no dimeric primers in PCR products.
Figure 2 RT-PCR analysis using Multicolor Real time PCR Detection system. (A) The real-time PCR amplification chart of iNOS. (B) The real-time PCR amplification chart of β-actin. (C) The real-time melt peak chart of iNOS. NC, normal control; DC, diabetic control; I, group of 50 mg kg− 1day− 1; II, group of 100 mg kg− 1day− 1; III, group of 200 mg kg− 1day− 1.
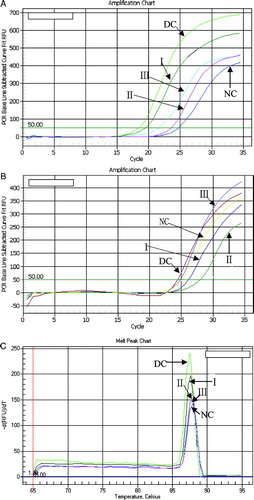
Figure 3 mRNA levels of iNOS in rat lens treated with different concentrations of HPS detected by gelose gel electrophoresis. 1, diabetic cataract control group; 2, 50 mg kg− 1day− 1 HPS-treated group; 3, 100 mg kg− 1day− 1 HPS-treated group; 4, 200 mg kg− 1day− 1 HPS-treated group; 5, normal control group. β-Actin expression at different treated groups is almost the same as that shown in the figure.

Table 4 The iNOS C.t., β-actin C.t. and opposite C.t.in different treatment groups.
Advanced glycation end products
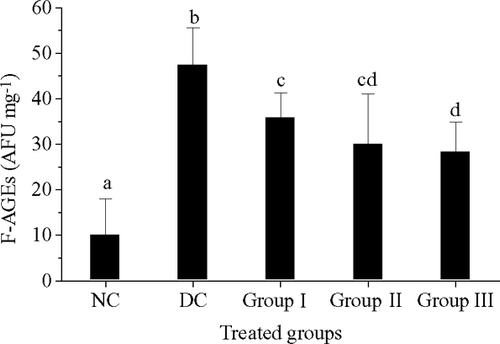
The fluorescence intensity of AGEs (F-AGEs) in different treated groups was calculated on a relative quantity of 1 mg mL− 1 fluorescence of hydroxyproline (). The fluorescence intensity of AGEs in normal control group was 10.23 AFU mg− 1 collagen and was significantly lower than that of the STZ treated groups. Compared with STZ groups, there were significant inhibitory responses of F-AGEs production in all treatments of HPS, which ranged from 34.9 to 40.2%.
Figure 4 The fluorescence intensity of AGEs (F-AGEs) in different treatment groups. Data are presented as the means ± standard deviations for three independent experiments. NC, normal control; DC, diabetic control. Group I, 50 mg kg− 1day− 1; group II: 100 mg kg− 1day− 1; group III, 200 mg kg− 1day− 1. The data with different letters in the same item indicate significant difference at p = 0.05.
Discussion
Recently, more and more scholars have a concept that cataracts as a complication of diabetes are likely to be induced by two distinct and separate types of post-translational modifications by diabetes (Keshore et al., Citation2003). First, it is well-known that metabolic changes brought about by diabetes increase production of reactive oxygen species (e.g., superoxide anions, hydroxy radicals and hydrogen peroxide) as well as reactive nitrogen species (e.g., nitroxyl anion [NO−], and peroxynitrite [ONOO−]) (Wolff et al., Citation1991; Dhalla et al., Citation2002; Evans et al., Citation2002). These free radical and nonradical species react with several amino acid residues, altering their structures and the tertiary structures of the parent protein. The synthesis of NO is mediated by a family of three NO synthase (NOS) isoenzymes encoded by different genes, which includes two types of Ca2 +-dependent enzymes (present in neurons and endothelial cells under the normal physiologic condition) and Ca2 +-independent (inducible isoform named iNOS, which expressed excessively under the pathologic conditions) (Raquel et al., Citation1999). The excessive expression of NO was induced by iNOS and then rapid response to O2 − in the environment to produce ONOO−, which is a stronger oxidant than others and gains in exponential multiple based on O2 − and NO. In the current study, the expression of iNOS in lens of HPS-treated animals was much lower than that in the diabetic control based on real-time PCR analysis. Quantitative analysis of NOS activity and NO production were also consistent with the result of RT-PCR. These results indicated that the polysaccharides from D. huoshanense. can antagonize oxidative stress, and one of the mechanisms might be the downregulation of iNOS gene expression.
Second, the elevation in circulating levels of aldose and ketose sugars brought on by diabetes accelerates the formation of Schiff bases on lysine, arginine, or histidine residues (nonenzymatic glycation reactions) (Wolff et al., Citation1991; Ulrich & Cerami, Citation2001). Over time (∼ 24–48 h), Schiff bases undergo internal rearrangement to form more stable Amadori products (Baynes et al., Citation1989). On long-lived proteins such as ryanodine receptors (Ferrington et al., Citation1998), Amadori products rearranged and further form the AGEs, which attached to protein to form the higher polymers, which resulted in the dysfunction of organisms (Brownlee et al., Citation1988; Bucala & Cerami, Citation1992; Soulis et al., Citation1997; Vasan et al., Citation2001). Compared with the rats treated with only STZ, we found the AGEs decreased in rat lens of HPS-treated groups, which indicated that reduction of AGEs formation was also a way to prevent cataract by HPS.
In conclusion, polysaccharides from D. huoshanense. could inhibit the oxidation pathway by downregulation of iNOS gene expression and AGEs formation to suppress the diabetic cataract. Because of this potential, it might be a new agent for prevention and cure of diabetic cataract in the future.
Acknowledgments
This work was financed by the Special Research Fund of Higher School's Doctoral Program from the Ministry of Education of China (no. 20060359006).
References
- Bao X F, Wang Z, Fang J N, Li X Y. Structural features of an immunostimulating and antioxidant acidic polysaccharide from the seeds of Cuscuta chinensis.. Planta Med 2002; 68: 237–243
- Baynes J W, Watkins N G, Fisher C I, Hull C J, Patrick J S, Ahmed M U, Dunn J A, Thorpe S R. The Amadori product on protein: Structure and reactions. Prog Clin Biol Res 1989; 304: 43–67
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissues and the biochemical basis of diabetic complications. N Engl J Med 1988; 319: 315–321
- Bucala R, Cerami A. Advanced glycosylation, chemistry, biology and implications for diabetes and aging. Adv Pharmacol 1992; 23: 1–34
- Dhalla N S, Temsah R M, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 2002; 18: 655–673
- Dubios M. Colormetric method for the determination of sugars and related substances. Anal Chem 1956; 24: 235
- Ettl A, Daxer A, Gottinger W, Schmid E. Inhibition of experimental diabetic cataract by topical administration of RS-verapamil hydrochloride. Br J Ophthalmol 2004; 88: 44–4
- Evans J L, Goldfine R D, Maddux B A, Grodsky G M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev 2002; 23: 599–622
- Ferrington D A, Krainev A G, Bigelow D J. Altered turnover of calcium regulatory proteins of the sarcoplasmic reticulum in aged skeletal muscle. J Biol Chem 1998; 273: 5885–5891
- Hao L, Mao Q, Ling Y. Gene expression and biochemical changes during the formation of rat diabetic cataract. Chin Ophthal Res 2005; 23: 297–300
- Keshore R B, Nallani K, Yu Y Q. Chronic diabetes increases advanced glycation end products on cardiac ryanodireceptors/calcium-release channels. Diabetes 2003; 52: 1825–1836
- Lin P, Bi Z M, Xu H. Advances in studies on pharmacology of plants from Dendrobium. Sw. Chinese Trad Herb Drugs 2003; 34: 19–22
- Palla S, Megha S, Tiruvalluru M. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci 2005; 46: 2092–2099
- Rahman K. Garlic and aging: New insights into an old remedy. Ageing Res Rev 2003; 2: 39–56
- Raquel M, Ana E, Pedro A. Androgen-dependent nitric oxide release in rat penis correlates with levels of constitutive nitric oxide synthase isoenzymes 1. Biol Reprod 1999; 61: 1012–1016
- Reidy A, Minassian D C, Desai P, Vafidis G. Increased mortality in women with cataract: A population based follow up of the North London Eye Study. Br J Ophthalmol 2002; 86: 424–42
- Soulis T, Thallas V, Youssef S, Gilbert R E, McWilliam B G, Murray McIntosh R P, Cooper M E. Advanced glycation end products and their receptors co-localise in rat organs susceptible to diabetic microvascular injury. Diabetologia 1997; 40: 619–628
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001; 56: 1–21
- Van Boekel M A, Hoenders H J. Glycation of crystallins in lenses from aging and diabetic individuals. FEBS Lett 1992; 314: 1–4
- Vasan S, Foiles P G, Founds H W. Therapeutic potential of AGE inhibitors and breakers of AGE protein cross-links. Expert Opin Invest Drugs 2001; 10: 1977–1987
- Vats V, Yadav S P, Biswas N R. Anti-cataract activity of Pterocarpus marsupium. bark and Trigonella foenum-graecum. seeds extract in alloxan diabetic rats. J Ethnopharmacol 2004; 93: 289–294
- Wolff S P, Jiang Z Y, Hunt J V. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 1991; 10: 339–352
- Zha X Q, Luo J P, Jiang S T. Induction of immunomodulating cytokines by polysaccharides from Dendrobium huoshanense.. Pharm Biol 2007; 45: 71–76
- Zha X Q, Luo J P, Shi W. Effects of metal ions on protocorm-like body proliferation in liquid culture of Dendrbium huoshanense. and plantlet regeneration. Acta Hort Sin 2006; 33: 179–181
- Zhao T F, Deng H C. Protective effect and mechanical study of sodium ferulate on kidneys in diabetic rats. Chongqing Medica University doctor degree study-thesis. 2002; 38–40
- Zoric L. Parameters of oxidative stress in the lens, aqueous humor and blood in patients with diabetes and senile cataracts. Srp Arh Celok Lek 2003; 131: 137–14
