Abstract
Bioassay-directed fractionation employing a multidrug-resistant (MDR) strain of Staphylococcus aureus. resulted in the isolation of an antibacterial prenyl substituted xanthone derivative (1) named hyperixanthone A from the root of Hypericum sampsonii. Hance (Hyperiaceae). Compound 1 showed promising inhibitory activity against the norfloxacin-resistant S. aureus. strain SA-1199B at a minimum inhibitory concentration (MIC) of 2 μ g/mL (4.3 μ M), wheres the positive standard antibacterial drug norfloxacin showed an MIC of 32 μ g/mL (100 μ M). This strain overexpresses the NorA multidrug efflux transporter, the major characterized drug pump in Staphylococcus aureus.. The activity of this compound against an effluxing strain of S. aureus. is reported here for the first time. Compound 1, together with 1,7-dihydroxyxanthone (2) and 2-hydroxyxanthone (3), were obtained by silica gel column chromatography, and their structures were determined by means of extensive NMR and MS spectra.
Introduction
Multidrug-resistant Staphylococcus aureus. infections, particularly those caused by methicillin-resistant S. aureus. (MRSA), have been a major threat to public health in hospitals and the community in the past decade. Despite the new advances in antibiotic development, MRSA infections remain a considerable concern due to the few agents that can be used in their treatment. In 2002, MRSA strains fully resistant to vancomycin were isolated in the United States (CDC, 2002). Resistance to linezolid has also been reported in some patients followed by prolonged antibiotic treatment in the United States (Peeters & Sarria, Citation2005). Therefore, there is an urgent need to develop new classes of antibiotics to fight the problem of drug resistance. In the search for antibacterial compounds from plants with activity against multidrug-resistant (MDR) Staphylococcus aureus., a number of species of the genus Hypericum. have been investigated because of their ability to produce extracts with antibacterial activity toward MDR strains (Gibbons et al., Citation2002; Mu et al., Citation2006).
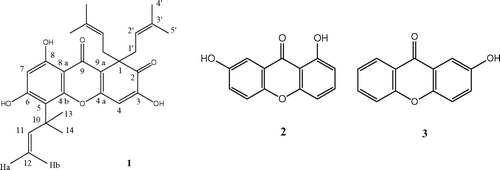
Our previous research in this area has led to the isolation and characterization of a number of antibacterial acylphloroglucinol natural products (Gibbons et al., Citation2002, Citation2005). In this paper, we have evaluated a Chinese species, Hypericum sampsonii. Hance (Hyperiaceae) of this group against a multidrug-resistant strain of Staphylococcus aureus.. The lower MIC (64 μ g/mL) of the root ethanoll extract against SA-1199B, a NorA overexpressing strain, prompted us to isolate the active constituents. This led to the isolation of the major active prenylated substituted xanthone derivative (1), hyperixanthone A (), which exhibited a minimum inhibitory concentration of 2 μ g/mL (4.3 μ M) against SA-1199B.
Materials and Methods
Plant and chromatography material
Hypericum sampsonii. was collected in September 2005 from Chalin County in Hunan province, China. The plant was identified by Dr. Zhang Wen-Ju, Associate Professor at the Center of Biodiversity of the Biology School, Fudan University, China. Voucher specimens (no. HS-001) have been deposited at the Natural Medicinal Chemistry Laboratory of the School of Pharmacy, Fudan University. The silica TLC (thin-layer chromatography) precoated plates and silica resin (200–300 mesh) for column chromatography were made in the Qingdao Marine Chemical Plant (QingDao, China).
Extraction and isolation
Air-dried and powdered (16 mesh) roots of the plant (1.1 kg) were extracted with 95% EtOH, affording 90 g of extract. This ethanol extract (90 g) was fractionated into various solvents of increasing polarity to afford the petroleum ether (40 g), methanol (13 g), and watersoluble fractions. The petroleum ether–soluble fraction was subjected to column chromatography over silica gel, eluting with a gradient from petroleum ether to ethyl acetate and final wash with methanol to afford 15 fractions (Fr. 1 to Fr. 15). Fraction 4 was re-chromatographed on a silica gel column with petroleum ether–acetone to yield 1 (100 mg). Fractions 7 and 8 were subjected to silica gel column chromatography using chloroform-acetone as eluent to give 2 (43 mg) and 3 (46 mg), respectively ().
Minimum inhibitory concentration (MIC) assay
SA-1199B was cultured on nutrient agar (Oxoid, Cambridge, UK) and incubated for 24 h at 37°C prior to MIC determination. The origin of SA-1199B is as described in Kaatz et al. (Citation1993). MICs were determined in duplicate by the microdilution assay as previously described (Gibbons & Udo, Citation2000). The control antibiotic norfloxacin was obtained from Sigma Chemical Co (Cambridge, UK). Mueller-Hinton broth (MHB; Oxoid) was adjusted to contain 20 and 10 mg/L of Ca2 + and Mg2 +, respectively. An inoculum density of 5× 105 CFU of S. aureus. strain SA-1199B was prepared in normal saline (9 g/L) by comparison with an 0.5 MacFarland turbidity standard. The inoculum (125 μ L) was added to all wells and the microtiter plate was incubated at 37°C for 18 h. For MIC determinations, 20 μ L of a 5 mg/mL methanol solution of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma) was added to each of the wells and incubated for 20 min. Bacterial growth was indicated by a color change from yellow to dark-blue. The MIC was recorded as the lowest concentration at which no growth was observed (Gibbons & Udo, Citation2000).
Results and Discussion
Compound 1 was isolated as yellow oil and gave rise to a molecular ion peak at m./z. 464 in its Electron Ionization Mass Spectrometry (EIMS) spectrum, which corresponded with a molecular formula C28H32O6. The IR spectrum for 1 showed the presence of a phenolic hydroxyl group (3347 cm− 1), a conjugated carbonyl group (1663 cm− 1), and an aromatic ring (1558, 1450 cm− 1).
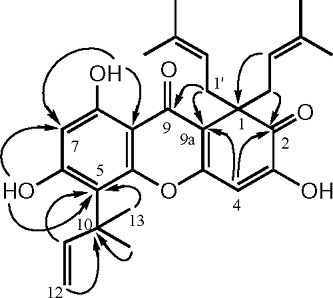
The 1H NMR spectrum of 1 () showed signals for a hydrogen-bonded hydroxyl (δ 13.42), two free hydroxyl groups (δ 7.14, s, OH-6; δ 7.01, s, OH-3), and two singlet aromatic protons at δ 6.48 (H-4) and δ 6.29 (H-7). Further signals in the 1H spectrum could be assigned to the presence of two identical isoprenyl substituents (C1′ to C5′), and a further 1,1-dimethyl-prop-2-enyl group (C10 to C14). In addition, the 13C NMR data indicated the presence of two carbonyl carbons (δ 201.2, C-2; δ 179.7, C-9), one aliphatic quaternary carbon (δ 55.8, C-1), and 10 aromatic carbons. In the HMBC spectrum, cross-peaks were observed between the protons of H3-13/14 and H-11 and the carbon of C-5, indicating that the 1,1-dimethyl-prop-2-enyl side chain was at C-5 (). The above data revealed that isolate was 1,2-dihydro-3,6,8-trihydroxy-1,1-bis.(3-methyl-but-2-enyl)-5-(1,1-dimethylprop-2-enyl)-xanthen-2,9-dione (1), previously isolated from H. erectum. Thunb. (Hyperiaceae, ex Guttiferae), and the NMR spectral data are in close agreement with those published (An et al., Citation2002) and later named as hyperixanthone A in this paper.
Table 1 NMR spectral data of compound 1 (CDCl3, J., Hz, 400 MHz).
Compound 2 was obtained as yellow needles. The Electrospray Ionization Mass Spectrometry (ESI-MS) of 2 suggested a molecular formula of C13H8O4 [M+H]+ (229.1). The 13C NMR spectrum displayed 13 signals in the aromatic region, including a signal for carbonyl carbon. The Distortionless Enhancement by Polarization Transfer (DEPT) 135 spectrum showed six positive signals, indicating that seven quaternary carbons were present and that methylene groups were absent. These signals were characteristic for a xanthone. The 1H NMR spectrum revealed three aromatic protons in an ABD spinning system with resonances at δ 7.54 (d, J.ortho = 9.0 Hz, H-5), δ 7.36 (dd, J. = 2.7, 9.0 Hz, H-6), and δ 7.43 (d, J. = 2.7 Hz, H-8) and three aromatic protons in an ABC spinning system with resonances at δ 7.04 (dd, J. = 0.8, 8.2 Hz, H-2), δ 7.70 (t, J. = 8.2 Hz, H-3), and δ 6.78 (dd, J. = 0.8, 8.2 Hz, H-4). Compound 2 was identified as 1,7-dihydroxyxanthone, and the NMR data for this compound were in close agreement with that in the literature (Lin et al., Citation1996; Shiu & Gibbons, Citation2006).
Compound 3 was obtained as yellow needles, and the ESI-MS of 3 suggested a molecular formula of C13H8O3 [M+H]+ (213.1). The 1H and 13C data for 3 were in close agreement with those published for 2-hydroxyxanthone (Luz Cardona, Citation1982).
All compounds were tested for their ability to inhibit the growth of SA-1199B. Compound 1 showed significant antibacterial activity with a MIC of 2 μ g/mL (4.3 μ M), and this compares well with the positive control norfloxacin, which gave an MIC of 32 μ g/mL (100 μ M), whereas compounds 2 and 3 were inactive. The greater degree of lipophilicity (and membrane solubility) for 1 may account for its antibacterial activity compared with 2 and 3. Xanthones are an underexploited class of antibacterial agent, and the fact that 1 demonstrates activity against an effluxing strain may indicate that this large molecule may not be a substrate for the NorA efflux pump. This characteristic would make prenylated xanthones worthy of further investigation as anti-staphylococcal drug leads.
1,2-Dihydro-3,6,8-trihydroxy-1,1-bis(3-methylbut-2-enyl)-5-(1,1-dimethylprop-2-enyl)-xanthen -2,9-dione (hyperixanthone A; 1.)
Yellow oil. Positive EI-MS m./z. (rel. int.): 464 [M]+ (4), 395 (100), 396 (32.7), 353 (31.8). IR (KBr) νmax 3347, 1663, 1640, 1594, 1558, 1502, 1450 cm− 1; UV (CHCl3) λmax (logε) 330 (2.95), 298 (3.27), 243 (3.29), 222 (3.05) nm; 1H,13C,1H-1H COSY, HMQC, and HMBC spectra of compound 4 are in close agreement with An et al. (Citation2002).
1,7-Dihydroxyxanthone (2.)
Yellow needles, m.p. 236–238°C. ESI-MS m./z.: 229.1 [M+H]+. The 1H NMR data are in good agreement with those reported by Lin et al. (Citation1996).13C NMR (400 MHz, CDCl3): δ 181.6 (C-9), 160.9 (C-1), 155.8 (C-4a), 154.1 (C-7), 149.3 (C-4b), 137.1 (C-3), 125.6 (C-6), 120.4 (C-8a), 119.3 (C-5), 109.6 (C-2), 107.8 (C-8, 8b), 107.1 (C-4).
2-Hydroxyxanthone (3.)
Yellow amorphous powder, m.p. 238–239°C. ESI-MS m./z.: 213.1 [M+H]+, IR, UV, and 1H NMR data are in close agreement with those reported by Luz Cardona (Citation1982). 13C NMR (400 MHz, CDCl3): δ 175.9 (C-9), 155.6 (C-4b), 153.9 (C-2), 149.2 (C-4a), 135.2 (C-6), 125.9 (C-8), 124.6 (C-3), 123.9 (C-7), 121.7 (C-8b), 120.4 (C-8a), 119.5 (C-4), 118.1 (C-5), 108.5 (C-1).
Acknowledgments
We are grateful for financial support from the National Science Foundation of China (30472068) and the Initiating Finance for Returnee of the Education Ministry of China (2006).
References
- An T Y, Hu L H, Chen Z L. A new xanthone derivative from Hypericum erectum.. Chin Chem Lett 2002; 13: 623–624
- CDC. Staphylococcus areus resistant to vancomycin. U.S. Morbidity and Mortality Weekly Reports 2002; 51: 565–567
- Gibbons S, Moser E, Hausmann S, Stavri M, Smith E, Clennett C. An anti-staphylococcal acylphloroglucinol from Hypericum foliosum.. Phytochemistry 2005; 66: 1472–1475
- Gibbons S, Ohlendorf B, Johnsen I. The genus Hypericum.—a valuable resource of anti-staphylococcal leads. Fitoterapia 2002; 73: 300–304
- Gibbons S, Udo E E. The effect of reserpine, a modulator of multidrug efflux pumps, on the in vitro. activity of tetracycline against clinical isolates of methicillin resistant Staphylococcus aureus. (MRSA) possessing the tet(K) determinant. Phytother Res 2000; 14: 139–140
- Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in staphylococcus aureus. Antimicrobial Agents and Chemotherapy 1993; 37: 1086–1094
- Lin C N, Chung M I, Liou S J, Lee T H, Want J P. Synthesis and anti-inflammatory effects of xanthone derivatives. J Pharm Pharmacol 1996; 48: 532–538
- Luz Cardona M, Seoane E. Xanthone constituents of Hypericum ericoides.. J Nat Prod 1982; 45: 134–136
- Mu Q, Gibbons S, Stavri M, Smith E, Zhou F S, Hu C Q. Antibacterial effects of Hypericum ascyron H. japonicum. against multidrug-resistant (MDR) Staphylococcus aureus.. Pharm Biol 2006; 44: 157–159
- Peeters M J, Sarria J C. Clinical characteristics of linezolid resistant Staphylococcus aureus. infections. Am J Med Sci 2005; 330: 102–104
- Shiu W KP, Gibbons S. Anti-staphylococcal acylphloroglucinols from Hypericum beanie.. Phytochemistry 2006; 67: 2568–2572