Abstract
The methanol extract of Psophocarpus tetragonolobus. (L.) DC (Leguminosae) root was screened for antimicrobial activity against Candida albicans. (Berkhout). The extract showed a favorable antimicrobial activity against C. albicans. with a minimum inhibitory concentration (MIC) value of 3.13 mg/mL. Apart from the fungicidal effects, imaging using scanning electron microscopy (SEM) was done to determine the major alterations in the microstructure of C. albicans.. The main abnormalities noted via SEM studies were the alterations in morphology and complete collapse of the yeast cells after 36 h of exposure to the extract. The effect of the extract on the growth profile of the yeast was also examined. It changed the normal growth profile of C. albicans., thus confirming the fungicidal effect of the extract on C. albicans.. In an acute toxicity study using rats, death was not observed, so the acute minimum fatal dose of the extract was greater than 2000 mg/kg, and we found no pathologic changes in macroscopic examination by necropsy of rats treated with extract. We conclude that the extract may be safely used as an antimicrobial agent.
Introduction
The past three decades have seen a dramatic increase in microbial resistance to antimicrobial agents (Baquero, Citation1997; Chopra et al., Citation1996) that has led to repeated use of antibiotics and insufficient control of disease (NCID, 2002). Hence, new antimicrobial agents are needed to deal with this situation. This encouraged us to evaluate the natural plant resources of our country as antimicrobial agents based on their ethnopharmaceutical use.
Among the many plants with healing properties in Malaysia, Psophocarpus tetragonolobus. (L.) DC (Leguminosae) has yet to gain importance and popularity. P. tetragonolobus. has commonly been used in traditional medicine for many years. The leaves are boiled to make a decoction applied as a lotion to cure smallpox (Perry, Citation1980). In the Shan states, the root is used as a poultice to treat vertigo (Burkill, Citation1935). In Indonesia, the leaf infusion is used as a wash for inflammation and suppurating sores and the leaves applied to cure boils. In New Guinea, on the other hand, the pod and edible tubers are considered roborant (Stopp, Citation1962). Leaves and seeds are eaten to cure skin sores such as boils and ulcers (Perry, Citation1980).
There is still much that is not known about P. tetragonolobus., and research must be conducted in many areas as available data are deficient on the status, extent, and utilization of P. tetragonolobus.. Our previous research showed that the extract of P. tetragonolobus. pods exhibited good antioxidant activity (Yoga Latha et al., Citation2005). The current study was carried out to determine the fungicidal activity of the extract of P. tetragonolobus. root against Candida albicans. (Berkhout) and reports the oral acute toxicity of the extract, which has never been reported in the literature.
Materials and Methods
Microorganism
Candida albicans. was used as the test organism and was obtained from a laboratory stock culture. The yeast was cultured on Sabouraud dextrose agar at 30°C for 24 h. The stock culture was maintained on Sabouraud dextrose agar slants at 4°C.
Plant collection and preparation of extracts
Fresh P. tetragonolobus. root was collected from Penang, Malaysia, in June 2006 and authenticated by the botanist of the School of Biological Sciences at Universiti Sains Malaysia, where the herbarium specimen was deposited. The sun-dried root was cut into small pieces, and 100 g of root was boiled in a Soxhlet apparatus with 300 mL methanol for 4 h. The root extract was evaporated to dryness in a rotary evaporator. The dried extract was then redissolved in 10% DMSO (v/v) to yield a solution containing 100 mg extract per milliliter.
Fungicidal activity
The fungicidal activity of the extract was determined following the method described by NCCLS (2002) with slight modifications.
Disk diffusion technique
The test microbe was removed aseptically with an inoculating loop and transferred to a test tube containing 5 mL sterile distilled water. Sufficient inoculums were added until the turbidity was equal to 0.5 McFarland (108 colony-forming units mL− 1) standard (bioMerieux, Marcy Petoile, France). One milliliter of the test tube suspension was added to 15–20 mL of Sabouraud dextrose agar before setting aside the seeded agar plate (9 cm in diameter) to solidify for 15 min. Nine Whatman's filter paper no. 1 disks of 6 mm diameter were used to screen the fungicidal activity. Each sterile disk was impregnated with 20 μ L of extract (corresponding to 100 mg/mL of crude extract); miconazole nitrate (30 μ g/mL, as positive control); 10% DMSO (v/v) (as negative control). The disks were placed on the surface of the seeded plates, incubated at 37°C overnight, and examined for zones of growth inhibition.
Determination of minimum inhibitory concentration (MIC)
A 16-h culture was diluted with a sterile physiologic saline solution [PS; 0.85% (w/v) sodium chloride] with reference to the 0.5 McFarland standard to achieve inoculums of approximately 106 CFU mL− 1. A serial dilution was carried out to give final concentrations between 1.563 and 200.00 mg crude extract per milliliter. The tubes were inoculated with 20 μ L bacterial suspension per milliliter nutrient broth, homogenized, and incubated at 37°C. After incubation, 50 μ L was withdrawn from each tube, inoculated on agar plates, and incubated at 37°C for 24 h. The MIC value was determined as the lowest concentration of the crude extract in the broth medium that inhibited the visible growth of the test microorganism.
Scanning electron microscope observations
Scanning electron microscope observations were carried out on C. albicans. cells. One milliliter of the C. albicans. cell suspension at a concentration of 1 × 106 cells per milliliter was inoculated on a Sabouraud dextrose agar plate and then incubated at 37°C for 12 h. The extract (2 mL), at a concentration of 100 mg per milliliter, was then dropped onto the inoculated agar and was further incubated for another 36 h at the same incubation temperature. A 10% DMSO–treated culture was used as a control. A small block of yeast containing agar was withdrawn from the inoculated plate at 0 and 36 h and fixed for scanning (Borgers et al., Citation1989).
Growth profile of C. albicans. in the presence of P. tetragonolobus. root extract
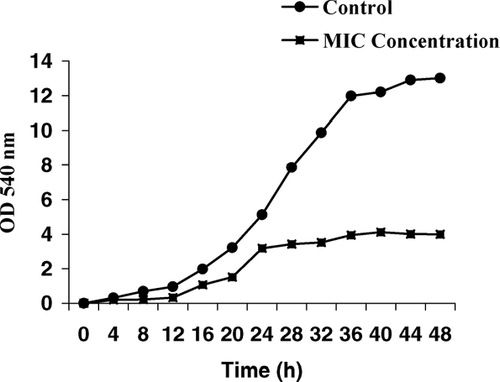
In order to assess the fungicidal effect with MIC concentration over time, growth profile curves were plotted. A 16-h culture was harvested by centrifugation, washed twice with phosphate saline, and resuspended in phosphate saline. The suspension was adjusted using the McFarland standard and was then further diluted in phosphate saline to achieve approximately 107 CFU mL− 1. Root extract was added to aliquots of 25 mL Mueller-Hinton broth (MHB) in a 50-mL Erlenmeyer flask (37°C) to achieve a concentration of 0 (control) and MIC (3.13 mg/mL), after addition of the inocula. Then, 1 mL inoculas were added to all Erlenmeyer flasks. Finally, 1 mL portions were removed and the growth of C. albicans. was monitored using this portion by measuring optical density at 540 nm (UV-9100; Ruili Co., Beijing, China). The growth of C. albicans. was measured every 4 h for 48 h by the above method.
Oral acute toxicity
In this assay, male and female rats (150–240 g) obtained from the animal shop, were used. The animals, housed in cages at 22°C, were starved overnight with free access to water. Two groups of 10 animals (control and test group), each containing an equal number of both males and females, were formed. A dose limit at 2000 mg/kg body weight of the extract dissolved in water was administered intragastrically to animals from the test group. The animals in the control group received water. In each case, the volume administered was 10 mL/kg body weight. After administration, the animals were closely observed during the first 3 h, and occasionally thereafter, for 14 days, for toxic signs and symptoms and death. The weight of the animals was measured daily. During the 14-day period, dead animals were autopsied, and, at the end of the period, survivors were sacrificed to examine vital organ gross changes.
Body weight evolution and weight of the organs from the control and the test groups were compared using the t.-test run on the software SPSS for Windows.
Results
Antimicrobial activity
The results of fungicidal activity of the extract against C. albicans. are given in . The extract exhibited a favorable activity against the yeast tested. The zone of clearance produce by the commercial antibiotic disk was larger than that produced by the extract disk (). The agar dilution method recorded the MIC value of 3.13 mg/mL.
Figure 1 Agar plate showing inhibitory zones: (A) crude extract (100 mg/mL), (B) miconazole nitrate (30 μ g/ mL), and (C) 10% DMSO (v/v).
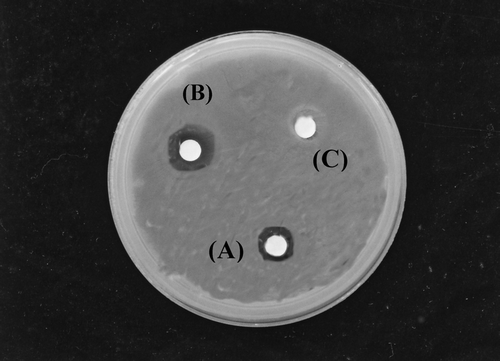
Table 1 Fungicidal activity (zone of inhibition and MICFootnotea.) of Psophocarpus tetragonolobus. root extract compared with commercial antibiotic miconazole nitrate.
Scanning electron microscope observation
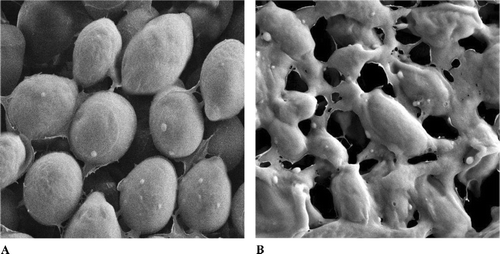
Cells treated with MIC of the crude extract underwent considerable morphologic changes in comparison with the control when observed under scanning electron microscope (). shows the SEM micrographs of the untreated and extract-treated cells of C. albicans. at 36 h exposure time. Untreated cells () showed many oval and smooth cells in appearance and some at a budding stage. After 36 h of exposure (), yeast cells completely collapsed and cavitated. It appeared that the cells had lost their metabolic functions completely.
Growth profile of C. albicans. in the presence of P. tetragonolobus. root extract
The growth profile for C. albicans. in Mueller-Hinton broth at MIC and 0 (control) concentration is shown in . The MIC was altering the normal growth profile for C. albicans. compared with the control (0 concentration). This finding confirmed the fungicidal effect.
Oral acute toxicity
No toxic symptoms or death was observed in any of the animals, and they lived up to 14 days. An autopsy at the end of the experimental period revealed no apparent changes in any organs. There were no changes either in the corporal weight or in the weight of the principal organs, and all animals exhibited a gain in body weight. shows the effect of the extract on the principal organ weights relative to body weight. In the daily consumption of food and water, water intake oscillated around 150 and 200 mL per day in females and males, respectively, in both groups. Food intake exhibited the same pattern of evolution in each sex and group. Therefore, the acute minimum fatal dose of the extract of P. tetragonolobus. root for rats is higher than 2000 mg/kg body weight.
Table 2 Effect of acute treatment of Psophocarpus tetragonolobus. root extract on the weight of the principal organs in rats.
Discussion and Conclusions
The fungicidal activities of P. tetragonolobus. root extract against C. albicans. examined in the current study, and its potency, were quantitatively assessed by the presence or absence of inhibition zone and zone diameter and MIC value. In this study, we particularly concentrate on dermatophytic fungi C. albicans. because the increasing prevalence of drug resistant C. albicans. recovered from hospitalized patients is a major concern worldwide (Bagg et al., Citation2003). Also, C. albicans. is the most abundant and significant species in human beings and known to be responsible for infections in people, and can cause vulvovaginitis, oral thrush, nosocomial infection, and candidiasis (Eggimann et al., Citation2003; Jarvis, Citation1995).
From this study, it appears that the extract exhibits a favorable antimicrobial activity against C. albicans.. This was confirmed by the alteration of the normal growth profile of C. albicans. by the extract. Furthermore, the SEM study showed that the extract could completely collapse the yeast cell and inhibit the growth of C. albicans.. Hence, C. albicans. infection could be treated with the extract, as the MIC for this yeast was found to be only 3.13 mg/mL. The acute minimum fatal dose of the crude extract was greater than 2000 mg/kg in rats, which is the single high dose recommended by OECD (Citation2000) guidelines for testing oral acute toxicity. Thus, our test suggested that P. tetragonolobus. root does not cause any apparent acute toxicity. The results of the current study concur with the use of this plant by native people for food preparation.
In addition, P. tetragonolobus. may find use as an antifungal agent in known dosages, especially in rural communities where conventional drugs are unaffordable or unavailable and the health facilities inaccessible. The results of the current study may suggest that P. tetragonolobus. root extract possesses compound(s) with antiyeast properties against C. albicans.. Further purification of the active compound(s) and in vivo. evaluation of antimicrobial activity of the extract against C. albicans. are therefore suggested for further studies.
References
- Bagg J, Sweeney M P, Lewis M A, Jackson M S, Coleman D, Baxter W, McEndrick S, McHugh S. High prevalence of non-albicans. yeasts and detection of anti-fungal resistance in the oral flora of patients with advanced cancer. Palliat Med 2003; 17: 477–481
- Baquero F. Gram-positive resistance: Challenges for the development of new antibiotics. J Antimicrob Chemother 1997; 39: 1–6
- Borgers M, Van De Ven M A, Van Cutsen J. Structural degeneration of Aspergillus fumigatus. after exposure to saperconazole. J Med Vet Mycol 1989; 27: 381–389
- Burkill I H. Dictionary of the Economic Products of the Malay Peninsula, 2nd ed. Crown Agents for the Colonies, London 1935; 2402, Edited by Ministry of Agriculture (Malaysia)
- Chopra I, Hodgson J, Metcalf B, Poste G. New approaches to the control of infections caused by antibiotic resistant bacteria. An industry perspective. JAMA 1996; 275: 401–403
- Eggimann P, Garbino J, Pittet D. Management of Candida species infections in critically ill patients. Lancet Infect 2003; 3: 772–785
- Jarvis W R. Epidemiology of nosocomial fungal infections, with emphasis on Candida. species. Clin Infect 1995; 20: 1526–1530
- NCCLS (National Committee for Clinical Laboratory Standards). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 3rd ed. 2002, Approved standard. NCCLS document M100-S12. Wayne, PA, NC-275 CLS
- NCID (National Center for Infectious Diseases). Campaign to Prevent Antimicrobial Resistance in Healthcare Settings. 2002, http://www.cdc.gov/drugresistacne/healthcare/problem.htm Centre for Disease Control and Prevention. Available at
- OECD (Organization for Economic Cooperation and Development). Guidelines for the Testing of Chemicals, Revised Draft Guideline: Acute Oral Toxicity. OECD, France 2000; 423
- Perry L M. Medicinal Plants of East and Southeast Asia. The MIT Press, Cambridge, MA 1980; 231
- Stopp K. The medicinal used by the Mt. Hagen people (mbowamb) in New Guinea. Econ Bot 1962; 17: 16–22
- Yoga Latha L, Sasidharan S, Zuraini Z, Saravanan D, Suryani S, Sangetha S, Siti Aishah M B. Antioxidant properties of Psophocarpus tetragonolobus.. J Trop Med Plants 2005; 6: 173–177