Abstract
In the Indian systems of medicine, there are many highly valued medicinal plants. Aegle marmelos. Correa (Rutaceae), commonly known as “bael,” is one of them. Its fruit exhibited antidiabetic, antihyperlipidemic, and antioxidant properties. This study was designed to evaluate in vitro. antioxidant activity of umbelliferone and psoralen in a methanol extract of A. marmelos. fruit pulp. A simple high performance thin layer chromatography (HPTLC) method has been developed for the simultaneous quantification of umbelliferone and psoralen. The method was validated for precision, repeatability, and accuracy in accordance with International Conference on Harmonisation (ICH) guidelines. Inhibition of oxygen-derived free radicals (ODFR), viz.., free radical scavenging, reducing power, superoxide anion scavenging assay, nitric oxide scavenging assay, and anti–lipid peroxidation, were carried out. All the antioxidant activities were compared with standard antioxidants such as BHA and α-tocopherol acetate. The extract was found to be a good scavenger of DPPH radical, nitric oxide, and superoxide anion with IC50 values of 1.015 ± 3.8, 1.381 ± 5, and 1.102 ± 4.1 mg/mL, respectively. The reducing power was calculated in terms of absorbance, which represented transfer of ferric ions to ferrous. Anti–lipid peroxidation was done with liver and brain homogenates, with IC50 values of 1.441 ± 5.2 and 1.628 ± 4.3 mg/mL, respectively. The results obtained from the current study suggest that A. marmelos. fruit is a potential source of natural antioxidants.
Introduction
Oxidative stress, induced by oxygen radicals, is believed to be a primary factor in various degenerative diseases and other conditions (Smith et al., Citation1996). Antioxidants can interfere with the oxidation process by reacting with free radicals, chelating free catalytic metals, and by acting as an oxygen scavenger (Sanchez, Citation2002). Many antioxidant compounds, naturally occurring from plant sources, have been identified as free radical or active oxygen scavengers (Dhalwal et al., Citation2005). Several Indian medicinal plants “rasayana.” have been extensively used in the Indian traditional system of medicine (Ayurveda) for the management of various diseases. Some of these have already been reported to possess strong antioxidant activity (Auddy et al., Citation2003). Recent investigations have shown that the antioxidant properties of various plants could be correlated with oxidative stress defense and in different human diseases. Among the various natural antioxidants, coumarin compounds are reported to be active, quenching oxygen-derived free radicals by donating a hydrogen atom or an electron to the free radical (Wanasundara & Shahidi, Citation1996).
Plants have played a significant role in maintaining human health and improving the quality of human life for thousands of years. Recently, interest has increased considerably in finding naturally occurring antioxidants in food or medicinal flora to replace synthetic antioxidants, which are being restricted because of their adverse side effects (Ito et al., Citation1983). Aegle marmelos. Correa (Rutaceae), commonly known as “bael,” is indigenous to India. It is also found in Myanmar, Pakistan, and Bangladesh. A literature survey showed that A. marmelos. has been used as food and for medicinal purposes for decades (Kirtikar & Basu, Citation1993). Some of the major chemical constituents of A. marmelos. categorized as coumarins include marmelosin, marmesin, psoralen, and umbelliferone (Kokate et al., Citation1990). The coumarins are phenolic compounds, and act as potent metal chelators and free radical scavengers. They are powerful chain-breaking antioxidants. A study revealed that umbelliferone demonstrates a promising protective effect against oxidative stress in heart and brain and also protects from the risk of diabetic complications (Kamalakkannan & Stanely, Citation2004; Wohaieb & Godin, Citation1987). Psoralen, when administered orally or topically followed by irradiation with long-wave ultraviolet radiation, proved to be an effective systemic treatment of psoriasis with well-characterized and controllable side effects (McNeely & Goa, Citation1998). A. marmelos. provides significant protection against chronic diseases, so there is a need to evaluate its in vitro. antioxidant activity and to standardize the plant material extract (Wagner & Bladt, Citation1996). In the past two decades, HPTLC has emerged as an efficient tool for the phytochemical evaluation of herbal drugs (Dhalwal et al., Citation2006; Milind et al., Citation2005). Considering the wide occurrence and therapeutic importance of umbelliferone and psoralen, in the current study, we have performed the antioxidant profile and developed a simple HPTLC method for the simultaneous quantification of umbelliferone and psoralen from A. marmelos. (fruit pulp).
Materials and Methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma Chemical Co. (Bangalore, India) potassium ferricyanide and sodium nitrite from Thomas Baker & Co. (Mumbai, India) trichloroacetic acid (TCA), butylated hydroxyanisole (BHA), sodium nitroprusside, ascorbic acid, and iron (III) chloride (FeCl3) from E. Merck (I) Ltd. (Mumbai, India) sulfanilamide from SISCO Research Labs. Pvt. Ltd. (Mumbai, India) N.-1-naphthylethylenediamine dihydrochloride from Unichem Ltd. (Pune, India) butylated hydroxytoluene (BHT), phenazine methosulfate (PMS), nitroblue tetrazolium (NBT), and thiobarbituric acid (TBA) from Hi-Media, α-tocopherol acetate from LOBA Chemicals (Pune, India) reference standard umbelliferone (purity 98%, w/w) and psoralen (purity 97%, w/w) were purchased from Natural Remedies Pvt. Ltd. (Bangalore, India). All other chemicals and reagents used were of analytical grade.
TLC conditions
HPTLC plate : 20 × 10 cm, 0.2 mm thickness precoated with silica gel 60 F254 (cat. no. 1.05548; E. Merck KgaA, Darmstadt, Germany).
Syringe: 100 μ L Hamilton (Bonaduz, Switzerland).
Spotter : Linomat V Automatic Sample Spotter (CAMAG, Muttenz, Switzerland).
Developing chamber : Glass twin trough chamber (20 × 10 × 4 cm) (CAMAG).
Densitometer : TLC Scanner 3 linked to WINCATS software (Muttenz, Switzerland).
Plant material
A. marmelos. (fruits) were collected from the Academy of Development Sciences, Kashale Post, Karjat, Maharashtra, during April 2006. The sample was authenticated and a voucher specimen was deposited in our Department of Pharmacognosy and Phytochemistry. Fruit pulp was dried and powdered to 40 mesh and stored in an air-tight container at 25°C.
Preparation of the extract
Accurately weighed fruit pulp of A. marmelos. (500 g) was extracted with methanol 3 times under reflux in water bath. The pooled extracts were combined and filtered through Whatman no. 1 filter paper. Dried extract (8.45 g) was obtained by concentrating filtrate under reduced pressure in a rotary evaporator.
Antioxidant activity
DPPH radical scavenging activity
The antioxidant activity, based on scavenging of stable DPPH free radical, was determined by the method described by Braca et al. (Citation2001). Different concentrations of test sample were added to 3 mL of a 0.004% methanol solution of DPPH. Absorbance at 517 nm was measured after 30 min, and the percent inhibition activity was calculated as [(A.0− A.1)/A.0] × 100 (where A.0 was the absorbance without sample and A.1 was the absorbance with sample).
Reducing power assay
The reducing power of extract was determined as reported (Oyaizu, Citation1986). Different concentrations of sample extract in 1 mL of methanol were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The reaction mixture was incubated at 50°C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10%) was added to the reaction mixture, which was then centrifuged at 3000 rpm for 10 min. To the upper layer of the solution (2.5 mL), distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%) were added. The absorbance of the reaction mixture was measured at 700 nm. Increased absorbance of the reaction mixture indicates increased reducing power.
Nitric oxide scavenging activity
The procedure is based on the principle that sodium nitroprusside in aqueous solution at physiologic pH spontaneously generates nitric oxide. Nitric oxide interacts with oxygen to produce nitrite ions, which were measured by Griess reaction. Scavenger of nitric oxide competes with oxygen, leading to reduced production of nitrite ions. The assay was performed according to the method described by Sreejayan and Rao (Citation1997). Different concentration of the sample extract were prepared in 100 mL phosphate-buffered saline, to which 0.1489 g sodium nitroprusside was added and incubated at room temperature. At different time intervals, 5.6 mL reaction mixture was taken out and 0.2 mL Griess reagent A (1% sulfanilamide in 5% phosphoric acid) was added. After 10 min incubation, 0.2 mL Griess reagent B (0.1% N.-1-naphthylethylene diamine dihydrochloride) was added and kept at 30°C for 20 min. Absorbance of the chromophore formed was measured at 542 nm against blank. The same reaction without the extract sample but equivalent amount of methanol serves as control. Concentration of nitric oxide was calculated from standard calibration of sodium nitrite solution of varying concentration.
Superoxide radical scavenging assay
The effect of superoxide radical production was evaluated using nitroblue tetrazolium reduction method (Nishikimi et al., Citation1972). The reaction mixture consisted of 1 mL NBT solution (156 μ M), 1 ml NADH solution (468 μ M), and 1 mL sample extract. The reaction was started by adding 100 μ L phenazine methosulfate solution (60 μ M PMS in phosphate buffer, pH 7.4) to the reaction mixture. The reaction mixture was incubated at 25°C for 5 min, and the absorbance at 560 nm was measured against blank. Decreased absorbance of reaction mixture indicates increased superoxide anion scavenging activity.
Anti–lipid peroxidation assay in rat liver and brain homogenate
Lipid peroxidation in rat liver and brain homogenate was evaluated by TBA method (Ohkawa et al., Citation1979; Sreejayan & Rao, Citation1994). The reaction mixture contained 0.5 mL (10%) rat liver homogenate, 1 mL, 0.15 M KCl, and 1 mL sample extract. Lipid peroxidation was initiated by adding 100 μ L 1 mM ferric chloride solution. The reaction mixtures were incubated for 30 min at 37°C. After incubation, the reaction was stopped by adding 2 mL ice-cold thiobarbituric acid solution containing 15% trichloroacetic acid, 0.38% thiobarbituric acid in 0.25 M HCl and 0.05% butylated hydroxytoluene. The reaction mixtures were heated at 80°C for 60 min, cooled, and centrifuged at 6900 rpm for 15 min. The absorbance of supernatant was measured at 532 nm against a blank, which contained all reagents except liver homogenate and drug. Identical experiments were performed to determine normal (without drug and FeCl3) and induced (without drug). The percentage of anti–lipid peroxidation effect (ALP %) was calculated by the formula:
Quantitative analysis using HPTLC
Preparation of calibration curves
The standard solutions of umbelliferone and psoralen were prepared (80 μ g/mL) in methanol. They were diluted to make different concentrations of calibration curves. The calibration range for umbelliferone was 1.6 to 4.8 ng and for psoralen was 16 to 96 ng. Each concentration of standard solutions of umbelliferone and psoralen (10 μ L) was applied in triplicate on TLC plates. The plates were developed in a solvent system of toluene-methanol (9.6:0.4 v/v) and scanned at 331 nm for umbelliferone and at 304 nm for psoralen. Calibration curves were prepared by plotting peak area versus concentration.
Preparation of sample solution
Fruit pulp of A. marmelos. (2.5 g) was extracted exhaustively with methanol (3 × 50 mL) under reflux for 1 h on water bath. The extracts were filtered and concentrated. The volume was made up to 50 mL in a volumetric flask with methanol.
Simultaneous quantification of umbelliferone and psoralen in drug sample
Suitably diluted sample solution (10 μ L) was applied in triplicate on a HPTLC plate along with standards. The plate was developed and scanned at 331 nm for umbelliferone and at 304 nm for psoralen. The peak areas and absorption spectra were recorded. To check the identity of the bands, the UV absorption spectrum of each standard was overlayed with the corresponding band in the sample track. The purity of the bands in the sample extract was checked by overlaying the absorption spectra at start, middle, and end positions of the bands. The amount of umbelliferone and psoralen in the sample was calculated using the linear regression equation derived from the calibration curves.
Statistical analysis
Data were analyzed by Student's t.-test. Experimental results were mean ± SD of five parallel measurements. p values < 0.05 were considered as statistically significant.
Results and Discussion
Aegle marmelos. is one of the widely used drugs in various Ayurvedic and Unani formulations for the treatment of various diseases. Certain plants show antioxidant activity because of their phenolic constituents. Coumarins, the secondary metabolites with varying molecular weight, are widely distributed in plants. The beneficial effects of coumarins are attributed to their antioxidant and chelating properties. Phytochemical analysis done by HPTLC densitometric analysis confirmed the presence of umbelliferone and psoralen in A. marmelos.. Umbelliferone and psoralen, categorized as coumarins, have good antioxidant activity. Of the various solvent systems tried, one containing toluene-methanol (9.6:0.4, v/v) gave the best resolution of umbelliferone (Rf. = 0.3) and psoralen (Rf. = 0.58) from the other components of the sample extract and enabled simultaneous quantification ( and ). The method was validated in terms of precision, repeatability, and accuracy (). The relationship between the concentration of standard solutions and the peak response was linear within the concentration range 1.6–4.8 ng/spot with a correlation coefficient of 0.997 for umbelliferone and 16–96 ng/spot with a correlation coefficient of 0.997 for psoralen. The percentage recovery at three different levels of umbelliferone was found to be 98.60%, 99.62%, and 98.44% with an average of 98.88%, and that of psoralen was found to be 99.99%, 100.71%, and 99.61% with an average of 100.104% (). Umbelliferone and psoralen contents found in the fruit pulp of A. marmelos. were 0.0070 ± 0.013% w/w and 0.0047 ± 0.009% w/w, respectively.
Figure 1 HPTLC chromatograms of fruit pulp of A. marmelos. extract along with standard umbelliferone and psoralen.
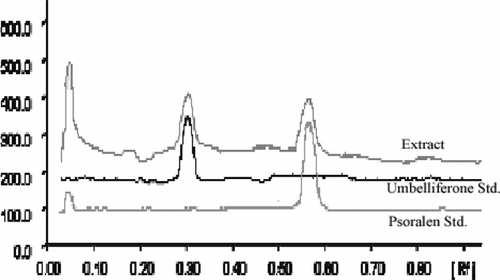
Figure 2 Overlay of UV absorption spectra of the marker compounds in the sample track with respective standards: (A) umbelliferone, (B) psoralen.
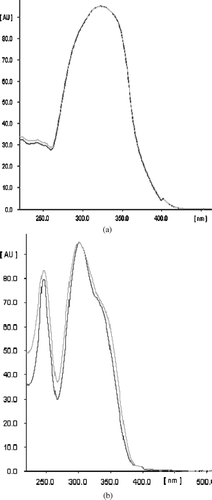
Table 1 Method validation parameters for the quantification of umbelliferone and psoralen by HPTLC method.
Table 2 Recovery study of umbelliferone and psoralen by the proposed HPTLC method.
As free radicals are potentially toxic, they are usually inactivated or scavenged by the human body's complex antioxidant enzyme defense systems or nonenzymatic antioxidant components before they can inflict damage to lipids, proteins, or nucleic acids (Halliwell & Gutleridge, Citation1989). However, when free radicals are generated in excess, or when the cellular antioxidant defense system is defective, there is need for dietary antioxidant to compensate. A. marmelos. fruit has been used as food and has good antioxidant activity against free radicals (Lee et al., Citation2003). A literature survey of A. marmelos. showed its activity against various ailments (Arul et al., Citation2005; Jagetia et al., Citation2005). Free radical scavenging activity was demonstrated by DPPH radical and nitric oxide scavenging assays. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule (Blois, Citation1958). It involves the reaction of specific antioxidant with a stable free radical DPPH. As a result, there is reduction in DPPH concentration by antioxidant, which decreases the optical absorbance of stable DPPH detected at 517 nm. Results represent the scavenging ability of A. marmelos. with an IC50 of 1.015 ± 3.8 mg/mL in comparison with α-tocopherol acetate and BHA with IC50 of 677.45 ± 2.5 μ g/mL and 42.95 ± 3 μ g/mL, respectively (). Nitric oxide scavenging activity was done by the method described by Sreejayan and Rao (Citation1997). The procedure is based on the principle that sodium nitroprusside in aqueous solution at physiologic pH spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions, which were measured by Griess reaction. Scavenger of nitric oxide competes with oxygen, leading to reduced production of nitrite ions. The NO scavenging property of the A. marmelos. extract showed significant results with an IC50 of 1.381 ± 5 mg/mL ().
Table 3 Effect of methanol extract of A. marmelos. fruit pulp on oxygen-derived free radical generation in vitro..
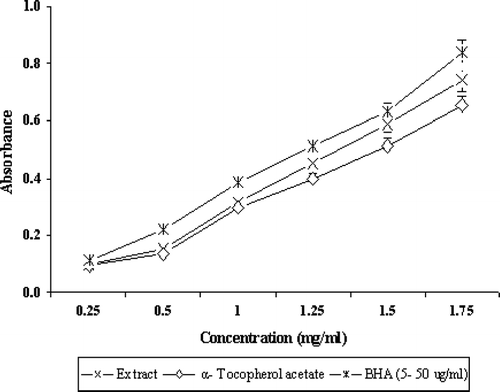
For the measurement of the reductive ability , Fe3 +-Fe2 + transformation in the presence of A. marmelos. extract was found and compared with α-tocopherol acetate and BHA. The reductive capability was found to increases with increases in concentration (). The activity of A. marmelos. extract against superoxide radical was of significance because superoxide can decrease the activity of other antioxidant defense enzymes such as catalase and glutathione peroxidase. Also, it can be cytotoxic by generating more reactive species such as peroxy nitrite (Oktay et al., Citation2003). Superoxide radicals are more detrimental because of their role as second messenger in fibroblast proliferation in inflammation and mediators of tissue destruction (Windrow et al., Citation1993). In the PMS-NADH-NBT system, drug possessing superoxide scavenging activity decreases the reduction of NBT, which is a measure of superoxide anion scavenging activity. A. marmelos. was found to scavenge the superoxide generated with an IC50 of 1.102 ± 4.1 mg/mL, whereas α-tocopherol acetate and BHA showed IC50 values of 577.45 ± 2.6 μ g/mL and 43.95 ± 3.1 μ g/mL, respectively ().
Figure 3 Reducing power of extract, α-tocopherol acetate, and BHA by spectrophotometric detection of Fe+ 3-Fe+ 2 transformations. Results are mean ± SD of five parallel measurements.
It is known that cleavage products of lipid peroxidation accumulate in the nervous system, the cardiac system, and muscle fibers (Nohl, Citation1993).Prevention of lipid peroxidation in rat liver and brain homogenate confirmed that it is active against effects of free radicals on biological membranes. The anti–lipid peroxidation effects of A. marmelos. extract in liver homogenate showed an IC50 of 1.441 ± 5.2 mg/mL, wherein brain homogenate showed an IC50 of 1.628 ± 4.3 mg/mL (). The result shows that inhibition of TBARS formation in rat liver and brain homogenate increased with increasing concentration.
Conclusions
The results from the current study clearly justified the use of A. marmelos. fruit pulp in various pathologic conditions. It gives protection against various free radicals by inhibiting DPPH radical, superoxide anion, nitric oxide, and decreasing the lipid peroxidation level. Different models used to evaluate the antioxidant activity suggest that A. marmelos. fruit is a good source of natural antioxidants. This may be considered as a conclusive correlation between Ayurvedic claims for this plant and relevant scientific studies.
References
- Arul V, Miyazaki S, Dhananjayan R. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos. Correa. J Ethnopharmacol 2005; 96: 159–163
- Auddy B, Ferreira M, Blasina F. Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol 2003; 84: 131–138
- Blois M S. Antioxidant determination by the use of stable free radical. Nature 1958; 181: 1199–1200
- Braca A, Tommasi N D, Bari L D, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis.. J Nat Prod 2001; 64: 892–895
- Dhalwal K, Biradar Y S, Rajani M. TLC densitometric method for simultaneous quantification of phyllanthin, hypophyllanthin, gallic acid and ellagic acid in Phyllanthus amarus. using HPTLC. J AOAC Int 2006; 89: 619–623
- Dhalwal K, Deshpande Y S, Purohit A P, Kadam S S. Evaluation of the antioxidant activity of Sida cordifolia. Pharm Bio 2005; 143: 754–761
- Halliwell B, Gutteridge J MC. Free Radicals in Biology and Medicine. Oxford University Press, Oxford 1989; 23, 130
- Ito N, Fukushima S, Hasegawa A, Shibata M, Ogiso T. Carcinogenicity of butylated hydroxyanisole in F344 rats. J Natl Cancer Inst 1983; 70: 343–347
- Jagetia G C, Venkatesh P, Baliga M S. Aegle marmelos. (L.) Correa inhibits the proliferation of transplanted Ehrlich ascites carcinoma in mice. Biol Pharm Bull 2005; 28: 58–64
- Kamalakkannan N, Stanely M P. Antidiabetic and anti-oxidant activity of Aegle marmelos. extract in streptozotocin-induced diabetic rats. Pharm Biol 2004; 42: 125–130
- Kirtikar K R, Basu B D. Indian Medicinal Plant. Vol. 1. International Book Distributor, DehradunIndia 1993; 309–310
- Kokate C K, Purohit A P, Gokhale S B. Pharmacognosy. Nirali Prakashan, PuneIndia 1990; 106–108
- Lee S E, Hwang H J, Ha J S, Jeong H S, Kim J H. Screening of medicinal plant extract for antioxidant activity. Life Sci 2003; 73: 167–179
- McNeely W, Goa K L. 5-Methoxypsoralen—A review of its effects in psoriasis and vitiligo. Drugs 1998; 56: 667–690
- Milind B, Srinivasa H, Harish P, Rajani M. A rapid densitometric method for simultaneous quantification of gallic acid and ellagic acid in herbal raw materials using HPTLC. J Sep Sci 2005; 28: 581–584
- Nishikimi M, Rao N A, Yagi K. The occurrence of super oxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 1972; 46: 849–853
- Nohl H. Involvement of free radicals in ageing: A consequence or cause of senescence. Br Med Bull 1993; 49: 653–667
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351–358
- Oktay M, Gülcin I, Küfrevioglu Ö I. Determination of in vitro. antioxidant activity of fennel (Foeniculum vulgare).. Lebensm Wiss Technol 2003; 36: 263–271
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 1986; 44: 307–315
- Sanchez C M. Methods used to evaluate the free radical scavenging activity in foods and biological system. J Food Sci Tech Int 2002; 8: 121–137
- Smith M A, Perry G, Sayre L M, Anderson V E, Beal M F, Kowall N. Oxidative damage in Alzheimers. Nature 1996; 382: 120–121
- Sreejayan Rao M NA. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol 1994; 46: 1013–1016
- Sreejayan Rao M NA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol 1997; 49: 105–107
- Wanasundara P K, Shahidi F. Optimigation of hexametaphosphate-assisted extraction of flaxseed proteins using response surface methodology. J Food Sci 1996; 61: 604–607
- Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas. Springer-Verlag, New York: Berlin Heidelberg 1996; 180
- Windrow V R, Winyard P G, Morris C J, Blake D R. Free radicals in inflammation: Second messengers and mediators of tissue destruction. Br Med Bull 1993; 49: 506–522
- Wohaieb S A, Godin D V. Alteration in free radical tissue defense mechanisms in streptozotocin diabetes in rats. Diabetes 1987; 36: 1014–1018
