Abstract
Aloe vera. L. (Aloeaceae) has been extensively studied for anti-inflammatory, antimicrobial, and cellular regeneration properties. This work evaluated in vivo. the effects of powder of freeze-dried Aloe vera. on rat pulp tissue. Pulp tissue was mechanically exposed to Aloe vera., and it was evaluated at 1 to 30 days after the procedure by histopathologic examination. A predominant acute-moderate inflammatory infiltrate was observed in the Aloe vera.–treated group 1 to 7 days after treatment. At 14 to 30 days, pulp tissue took a normal pattern when comparing Aloe vera. treatment with the Ca(OH)2 treatment; it was found that both treatment groups stimulated reparative dentin and the formation of complete bridge. Strong superficial necrosis was detected exclusively for Ca(OH)2. It seems evident that application of Aloe vera. in direct contact with the exposed pulp has acceptable biocompatibility and can lead to tertiary bridge formation.
Introduction
Changes in the dental care model show a tendency for the maintenance of the dental element and the preservation of its tissues. The traditional surgical management of caries is giving place to “conservative dentistry” (Anusavice, Citation1998). Conservative dentistry is based on a refined care model consisting of accurate caries diagnosis, the promotion of better oral health conditions, determination of disease risk, application of less-invasive therapies, and monitoring noncavitated lesions over time (Tziafas et al., Citation2000). In this context, the development of strategies to maintain vital pulp represents a great challenge for researchers.
Dentin and pulp are considered a single structure, denominated dentin-pulp complex, because of the intimate relationship between the peripheral pulp cell layer and its prolongation inside the dentinal tubules (Kitasako et al., Citation1999; Linde, Citation1985).
Nowadays, direct pulp capping is a therapeutic method aimed at treating reversible pulpar injury whenever dentin and pulp is affected by caries, restorative procedures, or trauma. Materials able to stimulate pulp tissue repair and tertiary dentin formation have been investigated extensively in pulp-capping situations (Koliniotou-Koumpia & Tziafas, Citation2005).
Calcium hydroxide, Ca(OH)2, is the choice material for conservative pulp treatment, including pulpotomy. It has been used for more than 50 years, showing a biological action mechanism that activates tissue enzymes that promote mineralization, which leads to the formation of dentinal bridge in approximately 90% of cases. The density of the newly formed tissue is variable, and in many cases it is difficult to observe in radiographic examination. Although the Ca(OH)2 has low resistance to compression, it promotes superficial necrosis, resulting in a transitory pulp inflammatory response, which has a short action time, and it is easily reabsorbed (Holland, Citation1971; Watts & Paterson, Citation1988).
Plants have been widely employed in folk medicine, mainly as a therapeutic resource to primary medicine (Reynolds & Dweek, Citation1999). Aloe vera. L. belongs to the Aloeaceae family and the Liliopsida class, like lily and garlic, and it has high water content, similar to cactus. It offers antimicrobial (Jasso de Rodríguez et al., Citation2005; Moody et al., Citation2004), anti-inflammatory (Poor et al., Citation2002), regenerative (Seyger et al., Citation1998), and nutritive properties (Vinson et al., Citation2005), of great value in medicine. The activity of Aloe vera. formulations is directly related to its physical-chemical properties, concentration, and the characteristics of the formulation, among other factors (Jasso de Rodríguez et al., Citation2005; Moody et al., Citation2004; Poor et al., Citation2002; Reynolds & Dweek, Citation1999; Seyger et al., Citation1998; Vinson et al., Citation2005). Aloe vera. has been used in folk medicine for many years, being empirically indicated for constipation, skin burns, rheumatic pains, and wounds. Aloe. is the expressed juice of the leaves of the plant. The use of Aloe vera. in pulp capping could offer biocompatible responses with a lesser degree of initial cytotoxicity as the inflammatory responses are necessary for a better regeneration of the reparative dentin.
The objective of this study was to compare pulp response and dentinal bridge formation in rats after pulp capping with Aloe vera. and calcium hydroxide.
Materials and Methods
Plant materials
Based on literature criteria (Villalobos & Salazar, Citation2001), a healthy plant of Aloe vera. L., approximately 4 years old, was selected from the UFMG Botanical Garden collection (Belo Horizonte, Brazil).
Preparation of the formulation
Leaves of Aloe vera. were collected, left to rest in distilled water for 8 h to eliminate aloine, and then were cut into pieces. Afterwards, pulp fragments were liquefied, sieved, filtered with negative pressure to obtain the juice, and freeze-dried. After that, they were sterilized by gamma ray and stored at 4°C in Eppendorf tubes.
Pulp capping
The animal model used herein was chosen following the protocol suggested by Sampaio (Citation1967) for pulp capping to minimize the risk of obtaining atypical response as recommended by International Standard Organization (1980).
Seventy-two noncarious upper first molars of Rattus norverguicus. (Wistar) (age, 4 weeks; weight, 250–300 g) were used for this experiment (two teeth of each animal) according to the experimental protocol approved by the animal experiment committee of UFMG, Brazil (076/04). The cavity was made under aseptic conditions and general anesthesia induced with 0.1 mL/100 g of intramuscular injection of 10 mL ketamine (100 mg/mL) mixed with 5 mL xylazine (20 mg/mL). The animals were divided into three groups: group 1, calcium hydroxide powder mixed with distilled water; group 2, freeze-dried Aloe vera.; group 3, water used as a negative control. A cavity was prepared on the occlusal phase of the second upper first molars of each animal. Crown access was made with spherical carbide burrs 1/4 (Maillefer, Switzerland) under isolation with a rubber dam and antisepsis with 70% alcohol and 0.3% iodine. The cavity was accessed using copious sterile water with low rotation. No additional retention was provided in the cavity preparation. In order to expose the pulp, a perforation was performed with a steel probe and gently cleaned with cotton buds and distilled water for 2 min. A metal foil interface (diameter, 2 mm) was used to prevent the amalgam influence into the pulp-capping procedure, and silver amalgam was used as a final restorative material (Velvalloy, S.S. White, Ltd., Brazil)
Histologic procedures
At 1, 7, 14, or 30 days postoperatively, the animals were sacrified with anesthetic overdose and cervical traction, and the maxillary molar specimens were dissected using a scalpel. The specimens were fixed in 10% formalin for 48 h at 4°C. Complete demineralization was obtained in 10% EDTA renewed every 4 days until the end point, followed by dehydration of the tissues in gradual ethanol series, and finally embedding in paraffin. The 5-μ m-thick sections thus obtained were stained with hematoxylin and eosin (H&E) and used to assess the amount of dentin formation and to evaluate the tissue response qualitatively and quantitatively. The results were evaluated according to the criteria of Standard histologic graduation and counting the number of cells in six fields (summarized in ).
Table 1 Standard histologic graduation.
Microscopic analysis was performed on the pulp-capping area by the same single blinded examiner using a light microscope. The fields were randomly selected and were submitted to cell counting in eight high-power fields (200×). The connective tissue interface in each case was randomly selected, and the percentage of positive cells for the total inflammatory infiltrate was obtained. Because the data did not conform to a normal distribution, a nonparametric analysis was used to compare both groups (ANOVA and Tukey test). The values were considered significantly different when the p value was < 0.05.
Results and Discussion
Group 1: Calcium hydroxide
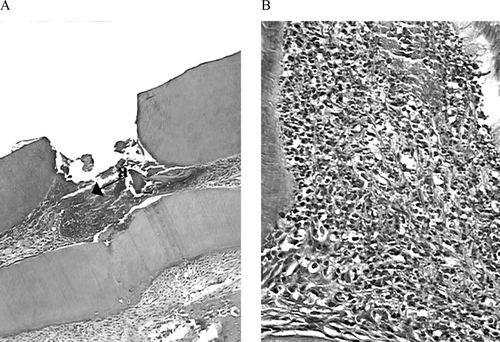
On the first day, the cavity floor was densely filled by granular structures scattered, and below there was a proliferation cellular zone. Superficial necrotic tissue was observed adjacent to the exposed site. A large number of neutrophils, enlarged capillaries, and few macrophages were observed adjacent to the necrotic tissue. The predominant acute infiltrate was slight and showed discrete necrosis, and debris cells were scattered in direct contact with the exposed area (). On the seventh day, the inflammatory infiltrate was slight and mixed with macrophages and cells like fibroblasts; predominant enhanced capillary and scarce giant cells were seen surrounding the marginal areas (). On the 14th day, slight inflammatory infiltrate with lympho-plasmocytes infiltrate predominated, and persistent enhanced capillary was noted. Exposure zone sealing by fibrous and mineral tissue was observed, but in some cases there was porosity formation. On the 30th day (), low inflammatory infiltrate took a normal pattern. Partial or complete dentin formation was observed in 87% of specimens, and complete sealing of the exposure area was observed in 30% of the cases.
Group 2: Aloe. vera.
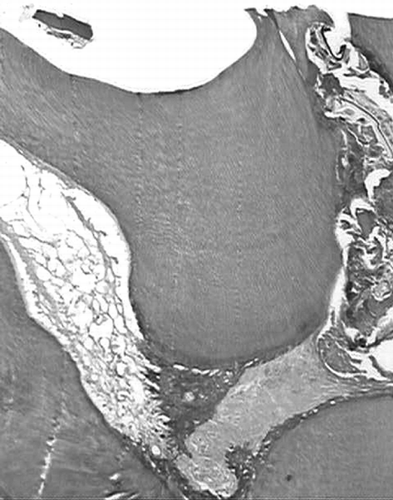
On the first day (group 2, freeze-dried Aloe vera.), the cavity floor was slight with granular structures scattered with red blood cells, and hemorrhage was observed in peripheral areas (). Superficial necrotic tissue was not observed in the marginal exposed site area. A large number of neutrophils such as fibroblasts cells, large cells, macrophages, and enhanced vessels were observed with predominant slight infiltrate (). The cell debris was scattered only in the superficial zone of the exposed area. The moderate mixed inflammatory infiltrate was observed on the seventh day. A large number of macrophages and enhanced vessels were observed, and giant cells were rarely found surrounding the material. During this period, the difference in inflammation grades between the Ca(OH)2 group and the Aloe. group was statistically significant (p. > 0.05). On the 14th day, the predominant infiltrate was slight, being composed of mostly fibroblast-like cells still with few macrophages and mononuclear infiltrate. At that moment, it was already possible to observe osteodentin formation mixed in fibrous tissue in some cases. However, the sealing of the exposure area was not complete. On the 30th day (), the formation of mineralized tissue, dentinal bridge, or osteodentin in 87% of the specimens with complete filling of the exposed sites was observed. Slight inflammatory infiltrate was noted in all specimens, and necrosis was not verified.
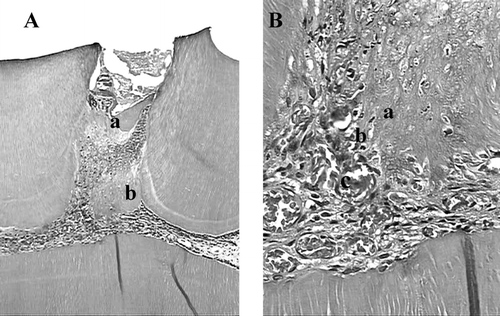
Figure 3 Histologic slide after pulp capping with Aloe vera L. in teeth of rat after first day. (A) magnified l0× (a) and (B) magnified 40× (b) Acute inflammatory infiltrate with red blood cells is present, and hemorrhage was observed in peripheral areas (H&E).
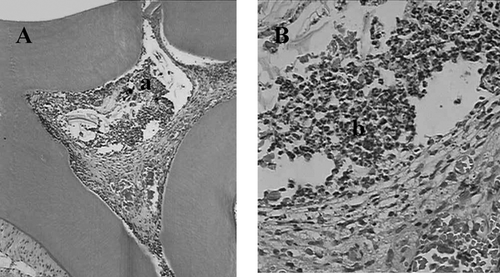
Figure 4 Histologic slide after pulp capping with Aloe vera L. in teeth of rat after 30 days. (A) magnified l0× (a) pulp exposure (b) mineralized tissue, (c) large vases. (B) magnified 40× (a) deeper layer of mineralized tissue, (b) odontoblastes layer reorganization, (c) capillary enhanced (H&E).
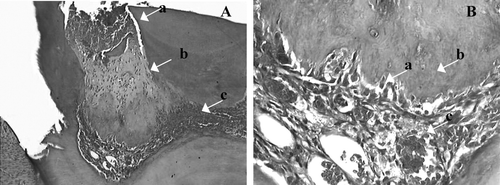
Table 2 Inflammatory cell response 1, 7, 14, and 30 days (n = 6) (macrophages, polymorphonuclear, mononuclear) with Aloe vera. and Ca(OH)2 after direct pulp capping.
Group 3: Control group
On the first day, the cavity floor was densely filled with granular structures with scattered red blood cells. Superficial necrotic tissue was observed adjacent to the exposed area. A large number of neutrophils and enhanced capillaries were observed; a few macrophages were observed. The inflammatory infiltrate was severe. On the seventh day, severe inflammatory infiltration involved the coronal pulp, enhanced capillaries, polymorphonuclear cells, macrophages, and giant cells. On the 14th day, the inflammatory infiltrate became even more intensive with lympho-plasmocyte infiltrate and persistent enhanced capillaries. Superficial necrosis area was observed adjacent to the exposed site in contact with calcium hydroxide. The inflammatory infiltrate remained intense on the 30th day with lympho-plasmocyte infiltrate. In two specimens, fibrous formation was observed, but complete exposure sealing was not observed (). The inflammatory response of the negative control group was statistically significant (P. > 0.05) when compared with Aloe. vera or calcium hydroxide at all periods.
Figure 5 Histologic slide of the negative control 30 days after pulp capping. Magnified 40×, pulp necrosis partial or total (5 μ m, H&E).
The intraexaminer performance evaluated by kappa analysis resulted in 0.807, which is considered an excellent agreement with the literature (Hunt, Citation1986) and perfect agreement (CitationEklud et al., 1986).
The evolution of pulp tissue response after capping follows serial events from inflammation to resolution, and in some cases the formation of dentin bridge. The literature reports that dentin formation can last up to 60 days. However, the most important factors in pulp capping are complete asepsis of the process and of the sealing of the cavity with filling material. In this work, we also confirmed the importance of the preservation of the restoration sealing after direct pulp capping and sealing with amalgam to obtain the formation of regular and complete barriers and pulp tissue regeneration as reported elsewhere (Isaia & Catanzaro-Guimarães, Citation1975).
Calcium hydroxide P.A. with distilled water has been used as a positive control over the area of exposure to evaluate the effect of this substance when chemically pure. Holland et al. (Citation1981) verified dentin bridge formation in 85% of the cases analyzed when they evaluated the effect of a mixture of Ca(OH)2 powder with water after 30 days. In agreement with this fact, greater success is attained when Ca(OH)2 is mixed with either distilled water or saline solution.
In the current study, the pulpal response after capping at the first day corroborates the results obtained with Ca(OH)2 by other authors (Holland et al., Citation1978; Estrela & Pesce, Citation1996). The initial superficial necrosis by coagulation shown in the Ca(OH)2 group, present in all cases, was more severe when compared with the Aloe vera. group. It suggests that the Aloe. composition allows a more physiologic pH (6.8) and would be better tolerated by the complex dentin-pulp. The application of Aloe vera. could mean decreased postoperative pulp signals, which are very common in pulp capping on the first day in humans.
In the current study, the initial severe inflammatory response was observed in all groups capped with Aloe vera. or Ca(OH)2. This response is expected to harmful stimuli, such as pathogens, damaged cells, or irritant injury; however, the resolution of the process happens in approximately 15 days. The intensity and duration of the inflammatory process has direct consequences on the stability and biocompatibility of the material (Kao & Lee, Citation2001).
The histology found on the seventh day is similar to the results with Ca(OH)2. In this period, repair zones formed by fibroblasts and collagen fibers was observed, whereas in the negative control group, the tissue presented disorganized characteristics of tissue degeneration with predominant necrosis. The acute inflammatory process results in decreased initial pH (Tziafas et al., Citation1995). This fact is relatively beneficial to the tissue, because inflammation stimulates enzyme release (alkaline phosphatase) and diverse factors that play an important role in the mineralization process.
The dentin bridge response could be considered excellent when compared with the results presented by other authors. Calcium hydroxide (Dycal) formed dentin bridge in 50% of the cases when compared with experimental, which caused severe pulp response (Heys et al., Citation1980). When the pulp response was analyzed after 14 days, there was an intense fibroblast proliferation with the tendency of isolating the exposed site and mineralized substance next to the exposed area was observed (Pitt Ford, Citation1980; Alle et al., Citation1984). However, the negative control group of the current study showed extensive necrosis, chronic moderate inflammatory infiltrate, enhanced capillaries, partial pulp tissue degeneration, and necrosis.
The therapeutic effect of Aloe vera. appears to be similar to that of Ca(OH)2. Dentin bridging was found in 87% of the teeth after pulp capping with Aloe vera.; although some reparative dentin had been formed in the other 13% of the cases, it was not considered as successful. In view of the complete bridge formation and the presence of compatible inflammation with the resolution process to health, the observed results support the claim that Aloe vera. is an effective material for capping exposed pulps.
Aloe vera. has a role in macrophage phagocytosis activity (Zhang & Tizard, Citation1996; Djeraba & Quere, Citation2000; Lee et al., Citation2001; Sun-Ai et al., Citation2005) and consequently in the resolution of the inflammation. Furthermore, mannose-6-phosphate has been shown to act as an anti-inflammatory agent and is present in the plant (Davies et al., Citation1994), which explains the lower inflammation in the Aloe vera. group at the first day. Besides, bioactive substances such as glycoprotein, polysaccharides, and β -sitosterol could stimulate wound healing, cell proliferation, and angiogenesis. Those facts may help to explain the proliferation of the fibroblast and dentin-like deposition found in the Aloe vera. group after 30 days.
Conclusions
The current study has shown that application of freeze-dried Aloe vera. in direct contact with the mechanically exposed pulp has acceptable biocompatibility and can lead to tertiary bridge formation.
Acknowledgment
This investigation was supported in part by a research grant from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and based on a thesis submitted to the graduate faculty, UFMG, in partial fulfillment of the requirements for the M.S. degree.
References
- Alle N, Costa N O, Pereira J C. Efeito de alguns materiais a base de hidróxido de cálcio em proteção direta de polpa de cães. Estomatol Cult 1984; 14: 21–28
- Anusavice K. Management of dental caries as a chronic infections disease. J Den Educ 1998; 62: 791–801
- Davies R H, Donato J J, Hartman G M, Hass R C. Anti-inflammatory and healing activity of a growth substance in Aloe vera.. J Am Podiatr Med Assoc 1994; 84: 77–81
- Djeraba A, Quere P. In vivo. macrophage activation in chickens with acemannan, a complex carbohydrate extracted from Aloe vera.. Int J Immunopharmacol 2000; 22: 365–372
- Eklud S A, Moller I J, Leclercq M. Calibrating examiners for oral health epidemiological surveys. World Health Organization, Geneva 1996, WHO/ORH/Epid. 93.1
- Estrela C, Pesce H F. Chemical analysis of the liberation of calcium and hydroxyl ions of calcium hydroxide pastes in connective tissue of the dog. Part I. J Braz Den 1996; 7: 41–46
- Heys D R, Heys R J, Cox C F, Avery J K. The response of four calcium hydroxides on monkey pulps. J Oral Pathol 1980; 9: 372–379
- Holland R. Histochemical response of amputed pulps to calcium hydroxide. Rev Brás Pesquisa Med Biologica 1971; 4: 83–95
- Holland R, Mello W, Nery M J, Souza V, Bernabé P FE, Otoboni Filho J A. Healing process of dog's dental pulp after pulpotomy and pulp covering with calcium hydroxide in podwer or paste form. Acta Odontol Pediatr 1981; 2(2)47–51
- Holland R, Souza V, Nery M J, Mello W, Bernabé P FE, Otoboni Filho J A. Effect of the dressing in root canal treatment with calcium hydroxide. Rev Facul Odontol Araçatuba 1978; 7: 39–45
- Hunt R J. Percent agreement, Pearsons correlation, and Kappa as measures of inter-examiner reliability. J Dent Res 1986; 65: 128–130
- International Standard Organization. Biological evaluation of dental materials. ISO, Genevra 1997; 54, ISO/TR 7405
- Isaia V G, Catanzaro-Guimarães S A. The formation of reparative dentin in dental pulps after protection with calcium hydroxide, formagen and zinc oxide and eugenol. Estomatol Cult 1975; 9: 265–270
- Jasso de Rodríguez D, Hernández-Castillo D, Rodríguez-García R, Angulo-Sánchez J L. Antifungal activity in vitro. of Aloe vera. pulp and liquid fraction against plant pathogenic fungi. Indust Crops Products 2005; 21: 81–87
- Kao W J, Lee D. In vivo. modulation of host response and macrophage behavior by polymer networks grafted with fibronectin-derived biomimetic oligopeptides: The role of RGD and PHSRN domains. Biomaterials 2001; 22: 2901–2909
- Kitasako Y, Arakawa M, Sonoda H, Tagami J. Light and scanning electron microscopy of the inner surfaces of resins used in direct pulp capping. Am J Dent 1999; 21: 217–221
- Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healty dog teeth with dentine adhesive systems. J Dent 2005; 33: 639–547
- Lee J K, Lee M K, Yun Y P, Kim Y, Kim J S, Kim Y S, Kim K, Han S S, Lee C K. Acemannan purified from Aloe vera. induces phenotypic and functional maturation of immature dendritic cells. Int J Immunopharmacol 2001; 1: 1275–1284
- Linde A. Cells and extracellular matrix of the dental pulp. J Dent Res 1985; 64: 523–529
- Moody J O, Adebiyi O A, Adeniyi B A. Do Aloe vera Ageratum conyzoides. enhance the anti-microbial activity of traditional medicinal soft soaps (Osedudu)?. J Ethnopharmacol 2004; 92: 57–60
- Pitt Ford T R. Pulpal response to MPC for capping exposures. Oral Surg Oral Med Oral Pathol 1980; 50: 81–88
- Poor M R, Hall J E, Poor A S. Reduction in the incidence of alveolar osteitis in patients treated with the SaliCept patch, containing acemannan hydrogel. J Oral Maxillofac Surg 2002; 60: 374–379
- Reynolds T, Dweck A C. Aloe vera. (L) leaf gel: A review update. J Ethnopharmacol 1999; 68: 3–37
- Sampaio P. Placement of a rubber dam on rat molars. J Dent Res 1967; 46: 11–02
- Seyger M MB, Van de Kerkhof P CM, Van Vlijmen-Willems I MJJ, De Bakker E SM, Zwiers F, De Jong E MGJ. The efficacy of a new topical treatment for psoriasis: Mirak. J Eur Acad Dermatol Venerol 1998; 11: 13–18
- Sun-Ai, Sun-Tack O, Sukgil S, Mi-Ran K, Dong-Seon K. Identification of optimal molecular size of modified Aloe. polysaccharides with maximum imunomodulatory activity. Int Immunopharmacol 2005; 5: 271–279
- Tziafas D, Alvanou A, Panagiotakopoulos N, Smith A J, Lesot H, Komnenou A, Ruch J V. Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo. implantation of dentin matrix components. Arch Oral Biol 1995; 40: 883–893
- Tziafas D, Smith A J, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent 2000; 28: 77–92
- Villalobos O C, Salazar C R. Efecto de un enjuague bucal compuesto de Aloe vera. en la placa bacteriana e inflamación gingival. Act Odontol Venezol 2001; 39: 1–17
- Vinson J A, Al Kharrat H, Andreoli L. Effect of Aloe vera. preparations on the human bioavailability of vitamins C and E. Phytomedicine 2005; 12: 760–765
- Watts A, Paterson R C. The responses of mechanically exposed pulp to prednisolone and triamnisolone acetonide. Int Endod J 1988; 21: 9–16
- Zhang L, Tizard I R. Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera. gel. Immunopharmacol 1996; 35: 119–128