Abstract
The current study evaluates the antibacterial and antifungal activities of extracts from different parts of Tagetes patula. Linn. (Asteraceae), reported for the first time in a single set of experiments. In the preliminary assay, the methanol extract of the flower (JFM) was found to possess antimicrobial activity against a number of bacteria with inhibition zone diameters ranging from 9 to 20 mm, the bioassay-guided fractionation of which led to the isolation of a flavonoid patuletin (3) in high yield as the active antibacterial principle with minimum inhibitory concentration (MIC) value of 12.5 μ g/disk against Corynebacterium. spp., Staphylococcus. spp., Streptococcus. spp., and Micrococcus luteus.. Its glucoside, patulitrin (4), was found to be weakly active, except against Staphylococcus saprophyticus., Streptococcus fecalis., and Streptococcus pyogenes. with inhibition zone diameters of 11, 16, and 12 mm, respectively. The cinnamate derivative (3b) of 3 showed antibacterial activity comparable with the parent flavonoid with a MIC value of 50 μ g/disk against Corynebacterium. spp., whereas benzoate derivative (3a) was found to be devoid of any activity; both the derivatives are new compounds. Moreover, the long-chain alcohol 5, which displayed antibacterial activity in the preliminary testing, was obtained in large quantity directly from the petroleum ether extract of the involucre of the flowers.
Introduction
Indiscriminate use of antibiotics, especially in the developing countries, often results in the development of resistant microbial strains (Gibbons, Citation1992). Pakistan is a developing and a densely populated country of South Asia. Like other countries, Pakistan is also facing a problem of drug resistance (Qudrat-e-Khuda, Citation2003). Several groups of scientists worldwide have focused their attention to develop strategies to combat the problem of emergence of drug-resistant pathogens (Burcher & Ho, Citation2001; Gibbons, Citation1992).
Medicinal plants have been known for their healing or disease-curing qualities for centuries. At the beginning of the 19th century, active secondary metabolites of medicinal plants were purified and used as a part of medicines. Several classes of natural products have been investigated for the source of antimicrobial entities (Copp, Citation2003; Cowan, Citation1999; Gibbons, Citation2004; Saeed et al., Citation1995; Tereschuk et al., Citation1997; Walsh, Citation2003; Wang et al., Citation1989).
Tagetes patula. Linn. (French marigold; Asteraceae), locally known as “jafri,” is grown in gardens for ornamental use across the globe. The plant and its different parts are known to possess biological activities, viz.., antiseptic, blood purifying, fly repellent, hepatoprotective (Vasilenko et al., Citation1990), insecticidal (Wells et al., Citation1993), nematicidal, and antidermatologic activities (Chadha, Citation1976; Kagan, Citation1991). Recently, the methanol extract of the whole plant (Mares et al., Citation2002) and its essential oil were claimed to be the possible source of antifungal agents (Sagar et al., Citation2002). The flower head is said to possess anthelmintic, antibacterial (Chadha, Citation1976), anti-inflammatory (Kasahara et al., Citation2002), carminative, diuretic, sedative, and stomachic properties and is also used in the preparation of a refreshing drink (Ivancheva & Zdravkova, Citation1993). The leaves are employed in kidney troubles and muscular pain, and the roots and seeds are used as purgative (Chadha, Citation1976). The chemical constituents of T. patula. include alkaloid (Faizi & Naz, Citation2002), benzofuran (Menelaou et al., Citation1991), flavonoids and their glycosides (Bhardwaj et al., Citation1980; Ivancheva & Zdravkova, Citation1993; Koloshina et al., Citation1980; Mashkovskaja & Dzuba, Citation2002), helenien (Tarpo & Cucu, Citation1961), polyacetylene (Kyo et al., Citation1990; Towers et al., Citation1984), steroids and terpenes (Kasprzyk & Kozierowska 1996; Vasudevan et al., Citation1997), and thiophenes (Kagan, Citation1991; Kyo et al., Citation1990; Mares et al., Citation2002; Margl et al., Citation2002). The two major classes of pigments present in Tagetes. are flavonoids and carotenoids. Flavonoids are natural products, and preparations containing these compounds as the principal physiologically active constituents have been used to treat human diseases for centuries (Cushnie & Lamb, Citation2005). Several plant-derived flavonoids have been reported to possess activity against multidrug-resistant bacteria, which are becoming common causes of infections in the acute and long-term care units in hospitals (Xu & Lee, Citation2001).
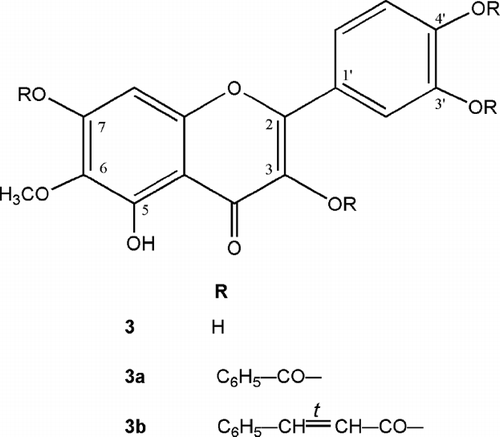
Two of the representative flavonoids from the flowers of T. patula. are patuletin (3) and patulitrin (4), which are very rare in nature (Abdel-Wahhab et al., Citation2005). The former was known to possess antioxidant (Schmeda-Hirschman et al., Citation2004), anti-inflammatory, antimicrobial (Saeed et al., Citation1995), antispasmodic, and hypotensive properties (Narayana et al., Citation2001). Both the flavonoids were also found to be nontoxic if administered in a dose of up to 100 mg/kg (Faizi et al., Citation2006a).
Careful review of literature revealed that no systematic work has been done on various parts of T. patula. for the evaluation of their antimicrobial activity, except for the aqueous extract of the flowers and essential oil of the aerial parts (flowers, leaves, and stem) (Chadha, Citation1976; Sagar et al., Citation2002). However, thiophenes, isolated from T. patula. and other species, were extensively studied for their phototoxicity against bacteria and fungi (Kagan, Citation1991; Mares et al., Citation2002; Romagnoli et al., Citation1994; Sagar et al., Citation2002). Additionally, the antimicrobial activity of flavonoids from leaves of T. minuta. L. has been reported, which revealed the presence of quercetagetin-7-arabinosyl-galactoside as the antimicrobial agent in the extract, and patuletin and patulitrin have also been detected in some fractions (Tereschuk et al., Citation1997). Considering these facts, evaluation of antimicrobial activity of different extracts and fractions from the flowers, foliage, and roots of T. patula. has been initiated and is described in this paper. The current investigation is a continuation of our efforts on the discovery of bioactive constituents from Tagetes. species (Faizi et al., Citation2006a; Saleem et al., Citation2004). The bioassay-guided isolation studies employing antimicrobial assay on different parts of T. patula., especially on flowers, in a single set of experiments have not been reported earlier.
Materials and Methods
General methods
UV (in MeOH) and IR (in CHCl3) spectra were recorded on Hitachi U-3200 (Hitachi, Japan) and JASCO A-302 (Japan) spectrophotometers, respectively. The EIMS and HREIMS spectra were recorded on Finnigan MAT 312 (Finnigan, Germany) and JMS HX-110 (Jeol, Japan) spectrometers. The 1H and 13C NMR spectra were run on a Bruker AV-300 (Biospin) instrument operating at 300 MHz and 75 MHz, respectively. The chemical shifts and coupling constant (J.) are shown in (δ) ppm and Hz. Silica gel HF 60254 was used for VLC and PTLC. All the chemicals and solvents used were of analytical grade. Benzoic anhydride and cinnamoyl chloride were purchased from Merck (Hohenbrunn, Germany). The physical data of the compounds isolated and prepared were identical with those reported in the literature (Coogan et al., Citation1965; Chan et al., Citation1975; Buckingham, Citation1994; Miyazawa et al., Citation2003; Schmeda-Hirschmann et al., Citation2004).
Plant material
Seedlings and the whole plant of Tagetes patula. were collected from the campus of the University of Karachi during the years 2000 and 2003. The plant was identified by Dr. Rubina Dawar, Department of Botany, University of Karachi, and the voucher specimen (no. 67280) was deposited in the herbarium of the same department.
The plant was divided into its various parts, i.e., aerial part (leaves, stem), flowers, roots, involucres, and seeds. Seedlings of three different sizes (I = 11 cm, 7 g; II = 13 cm, 7 g; III = 21 cm, 9 g) were also collected from the same site.
Bioassay-directed isolation work on flowers
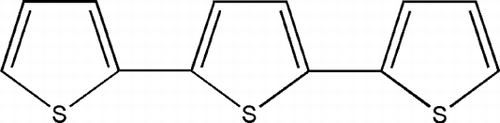
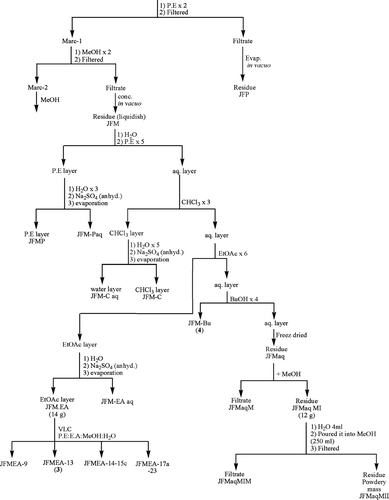
Air-dried, uncrushed, orange-red flowers (750 g) of T. patula. were successively extracted for 48-h at room temperature (∼ 27°C) twice with petroleum ether (P.E.) and methanol. The extracts were evaporated separately using a rotary evaporator (60–65°C) to yield their respective dry P.E. residue (JFP, 691 g) and methanol residue (JFM, 300 g), respectively, and screened for antimicrobial activity. The extract JFP, which showed good activity against Gram-negative bacteria, has a prominent spot on TLC (Rf. = 0.45, silica gel 60F254, P.E.) which was separated through PTLC (silica gel HF 60254, P.E.) and identified as α -terthienyl (1) (Chan et al., Citation1975). The gummy methanol extract (JFM) was found to be the most active and accordingly selected for bioassay-guided fractionation using the solvent-solvent extraction method. Thus, the extract (JFM) was partitioned between distilled water and petroleum ether to give P.E. phase (JFMP), and the aqueous layer was subsequently treated with chloroform (three-times, JFM-C), ethyl acetate (six-times, JFM-EA), and butanol (four-times, JFM-Bu). Each phase (P.E., CHCl3, EtOAc, except BuOH) was washed with water and dried over anhydrous sodium sulfate. As the six EtOAc phases showed more or less the same spots on TLC, they were combined and solvent was removed in vacuo. affording a residue (JFM-EA, 14 g), which was found to be the most active against various microbes. Hence, it was subjected to vacuum liquid chromatography (VLC) with silica gel as adsorbent whereby 23 fractions were obtained. JFMEA-13 possessed the active antimicrobial compound, patuletin (3) (Kaloshina & Mazulin, Citation1983). The remaining eluates, JFMEA-14a-15c and JFMEA-17a to 23, were combined together separately on the basis of TLC. The former set (JFMEA-14-15c) exhibited very strong antibacterial activity, and it is under chemical consideration. The butanol phase (JFM-Bu), which contained a pure compound, patulitrin (7-O.-glucoside of patuletin, 4), was found to be less active than the ethyl acetate phase (JFM-EA) (Tereschuk et al., Citation1997). The aqueous phase, after butanol extraction, was freeze-dried giving a residue (JFMaq) that was treated with methanol affording methanol soluble (JFMaqM) and insoluble (JFMaqMI) fractions. In order to separate polysaccharides from JFMaqMI, it was dissolved in 10 mL water and poured drop-by-drop into 250 mL methanol, thereby, two fractions JFMaqMIM (methanol soluble) and JFMaqMII (methanol insoluble), were obtained. They are under chemical investigation. All the fractions (JFMaqM, JFMaqMI, JFMaqMII) and JFMaq itself were found to be inactive as antimicrobial agents.
Extraction of aerial parts, roots, and seeds
Roots (JR), seeds (JS), and aerial parts (JW) were successively extracted with petroleum ether (P), methanol (M), and 70% methanol (M70). These extracts were evaporated under reduced pressure affording respective gummy residues, JRP, JRM, JRM70; JSP, JSM, JSM70; and JWP, JWM, JWM70.
Involucres
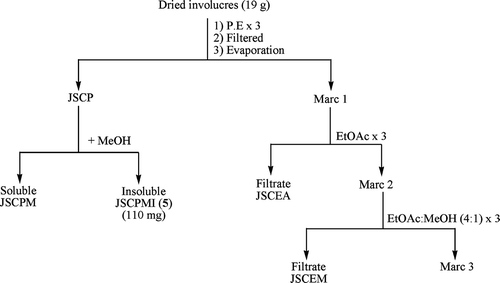
Dried involucres (19 g) without achenes of T. patula. were extracted three-times with P.E. at room temperature. The P.E. extracts were combined together and freed of the solvent in vacuo. to give a residue JSCP, which on treatment with methanol furnished methanol soluble (JSCPM) and insoluble white pure crystals, JSCPMI (110 mg). The UV, IR, EIMS, 1H NMR, GC, and GC/MS spectral data identified the structure of JSCPMI as a long-chain primary alcohol, tetracosanol (5) (Buckingham, Citation1994). The marc left after extraction with P.E. was percolated three-times with ethyl acetate followed by ethyl acetate: methanol (4:1). These extracts were freed of the solvent separately in vacuo. to afford residues JSCEA and JSCEM, respectively ().
Seedlings
Three different sizes of seedlings of T. patula. (I = 11 cm, 7g; II = 13 cm, 7g; III = 21 cm, 9 g) were extracted separately with P.E. at room temperature. The P.E. extracts were freed of the solvent in vacuo. giving the residues JSdPI, JSdPII, and JSdPIII, respectively. The respective marcs that were obtained after filtration of P.E. were further extracted three-times with ethyl acetate and methanol, affording ethyl acetate (JSdEAI, JSdEAII, JSdEAIII) and methanol (JSdMI, JSdMII, JSdMIII) extracts.
Derivatization of patuletin (3)
3,3′,4′,7-Tetrabenzoylpatuletin (3a)
Patuletin (100 mg) in pyridine (0.5 mL) was treated with benzoic anhydride (0.2078 g), and the reaction mixture was kept at room temperature for 3 days, which on evaporation of pyridine was divided into petroleum ether soluble and insoluble portions. The latter on crystallization with chloroform and methanol (1:1) furnished yellow shiny crystals (Pat-Bz) exhibiting a single spot on TLC (Rf. = 0.54, silica gel 60 F254, P.E.:EtOAc, 6.7:3.3); yield 85 mg (37.8%), m.p. 204°C. Spectral studies (MS, UV, IR, 1H NMR, 13C NMR, 2D NMR) characterized its structure as 3,3′,4′,7-tetrabenzoylpatuletin (3a) (Faizi et al., Citation2006b).
3,3′,4′,7-Tetracinnamoylpatuletin (3b)
Following is a revised procedure in which patuletin and cinnamoyl chloride were taken in the proportion of 1:30 (mole ratio) resulting in the formation of (3b) as a major product in a shorter time period (< 1 h) than the 10 days required in the previous procedure (Faizi et al., Citation2006a). Patuletin (0.1002 g) in pyridine (2.5 mL) was treated with cinnamoyl chloride (1.503 g) and kept at room temperature for about 1 h. The TLC profile showed the formation of one major product with some minor ones in the reaction mixture. The major product formed was precipitated out as off-white powder by pouring the reaction mixture into methanol (∼ 100 mL). It was filtered and recrystallized with CH2Cl2 and MeOH (1:1) affording fine crystals (PC), which showed a single spot on TLC (Rf. = 0.44, silica gel 60F254, P.E.:EtOAc, 6.7:3.3). Very slow conversion of 3b into its hydrolyzed products at room temperature was observed; yield 0.125 g, 57.6%. Spectral studies (MS, UV, IR, 1H NMR, 13C NMR, 2D NMR) identified its structure as 3,3′,4′,7-tetracinnamoylpatuletin (3b) (Faizi et al., Citation2006b).
Biological data
Microorganisms used
All bacterial cultures used are listed in , , , .
Table 1 Antibacterial activity of different extracts of aerial parts, roots, and seeds of Tagetes patula. plant.
Table 2 Antibacterial activity of different extracts, fractions, pure compounds patuletin (3) and patulitrin (4) from flowers of Tagetes patula., and the derivatives 3a, 3b.
Table 3 Antibacterial activity of involucre, seedlings, and pure compound (5) of Tagetes patula..
Table 4 The MIC (μ g/disk) of fractions and pure compounds.
Determination of antibacterial activity
Antibacterial activity of all the test samples was determined by the disk diffusion method (Bauer et al., Citation1966) on iso-sensitest agar (BioM, Cerritos, USA) plate. Briefly, 6-mm sterile filter paper disks containing 200 μ g of test sample were prepared from stock solution prepared in DMSO. The disks were air-dried and then placed on a lawn of the test organisms. The plates were incubated at 37°C for 24 h followed by the measurement of the zones of inhibition.
Determination of antifungal activity
Antifungal activity was also determined by the disk diffusion method (Bauer et al., Citation1966), as described earlier, with a difference that fungal cultures were seeded on Sabouraud's dextrose agar (Oxoid, Baringstoke, Hampshire, England) and incubated at 30°C for 7 or 14 days, as needed. Fungal cultures used during this study are listed in .
Table 5 Antifungal activity of different extracts, fractions, and pure compound from Tagetes patula. plant.
Determination of minimum inhibitory concentration (MIC)
The test samples showing zones of inhibition of 10 mm or more diameter were considered significant and were selected for the determination of their MICs by the disk diffusion method (Bauer et al., Citation1966). Recent studies suggest that the agar diffusion method is comparable with the broth dilution method for MIC determination. This method is effective not only against bacteria (CitationAndrews et al., 1991) but also against fungi (Serrano et al., Citation2004; Lopez-Oviedo et al., Citation2006).
Sterile disks containing varying concentration of samples (from 0.1 to 100 μ g/disk) were prepared. The prepared disks were placed onto the surface of iso-sensitest agar plate seeded with test culture. Similarly, MIC was also determined against fungi using 0.1–200 μ g/disk. The MIC was determined as the lowest concentration of the sample showing the zone of inhibition.
Results and Discussion
Antibacterial activity
In the current work, crude petroleum ether, methanol, and 70% methanol extracts of different parts (aerial, roots, and seeds) of T. patula. were tested against 18 Gram-positive and 12 Gram-negative bacteria (, , , ). Amongst the three extracts of aerial part, the petroleum ether extract (JWP) was active against Bacillus cereus, Bacillus subtilis, Micrococcus luteus, Micrococcus lysodeikticus, Staphylococcus aureus, Staphylococcus saprophyticus, Streptococcus pyogenes., and all the Gram-negative bacteria except Pseudomonas aeruginosa. PAO 286. The methanol (JWM) and 70% methanol extract (JWM 70) showed significant activity against Klebsiella pneumoniae. and Pseudomonas aeruginosa. PAO 286, respectively. Petroleum ether extract of roots (JRP) showed significant inhibitory effect against Bacillus subtilis. and Pseudomonas aeruginosa. PAO 286, M. luteus. and Streptococcus pneumoniae. were significantly sensitive to the methanol extract (JRM), whereas 70% methanol extract (JRM 70) possessed very weak activity against all the bacteria. The three seed extracts (JSP, JSM, JSM70) exhibited good activity against Bacillus subtilis. and Shigella flexneri.. JSM-70 was also effectively active against Proteus mirabilis. ().
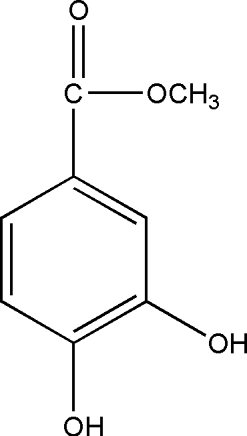
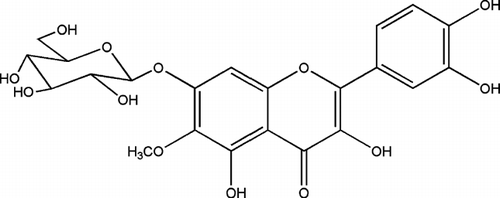
Flowers of T. patula. were extracted with petroleum ether and then with methanol. The main component of the petroleum ether extract (JFP) has already been reported and found to be α -terthienyl (1) () (Kagan Citation1991; Romagnoli et al., Citation1994). JFP was highly active against Pseudomonas aeruginosa. PAO 286, and Proteus vulgaris. (). Being significantly active against most of the Gram-positive bacteria, the methanol extract of flowers (JFM) was further divided through solvent-solvent extraction into different fractions (). Amongst these, only the ethyl acetate portion (JFM-EA) showed promising activity against most of the Gram-positive bacteria and one Gram-negative organism, Shigella flexneri. (). It was further fractionated, employing vacuum liquid chromatography, affording 23 fractions. VLC fraction-9 (JFMEA-9), containing methyl protocatechuate (2) () and an unknown compound, was almost inactive. Fraction (JFM EA-13), containing a pure compound patuletin (3) (), was effective against almost all Gram-positive bacteria tested (). Most of the Gram-positive bacteria were also significantly inhibited by the fraction JFMEA-14-15c, which consisted of a mixture of compounds and is under chemical investigation. The fraction JFM-Bu, containing 7-O.-glucoside of patuletin (patulitrin, 4) (), was a potent inhibitor of Streptococcus faecalis., Streptococcus pyogenes., and Staphylococcus saprophyticus. ().
Scheme 2 Bioassay-guided isolation of antimicrobial principle (3) and (4) from fresh, air-dried uncrushed, orange-red flowers of Tagetes patula. (750 g).
In the current studies, patuletin (3) emerged as the major flavonoid from flowers of T. patula., possessing antibacterial activity against Bacillus. spp., Micrococcus. sp., Staphylococcus. spp., and Streptococcus. spp. Therefore, it was subjected to structure-activity relationship (SAR) studies, which resulted in the preparation of two new derivatives, 3,3′,4′,7-tetrabenzoylpatuletin (3a) and 3,3′,4′,7-tetracinnamoylpatuletin (3b). The antibacterial activity of 3b, as shown in , was more or less similar to that of its parent compound, patuletin (3), against Gram-positive bacteria, and it was fractionally better against Gram-negative bacteria (). On the other hand, Gram-negative bacteria did not show any susceptibility to 3,3′,4′,7-tetrabenzoylated derivative (3a) (). It is imperative to note that cinnamic acid (CA) was reported as a well-known antimicrobial compound (Cowan, Citation1999). In the current study, antimicrobial activity of CA as well as benzoic acid (BA) was also evaluated, which revealed that BA was relatively more active than CA. However, the introduction of benzoyl and cinnamoyl groups in patuletin derivatives (3a and 3b, respectively) did not result in any positive change on its antimicrobial profile, but rather a negative effect was noted against bacteria (). Patulitrin (4), a monosubstituted (7-O.-glucosyl) derivative of 3, and another pure flavonoid obtained from the flowers extract was also inactive against most of the tested organisms. Antimicrobial activity of several patuletin derivatives, as reported earlier and in the observations made during this study, led to the conclusion that it is not possible to establish a generalized structure-activity relationship on the bioactivity (Tereschuk et al., Citation1997; Wang et al., Citation1989), as the inhibition of bacteria is a multifactoral phenomenon.
Moreover, the extracts of dried involucres (without achenes) were also evaluated for antimicrobial potential. The petroleum ether extract (JSCP) exhibited significant antibacterial activity against some of the test organisms (), which on treatment with methanol afforded white pure crystals (JSCPMI). Spectroscopic studies revealed that JSCPMI was a long-chain alcohol tetracosanol (5) (), showing significant activity against Bacillus stearothermophilus. NCTC 10003. The ethyl acetate extract (JSCEA) was more active against Bacillus subtilis. and Salmonella paratyphi. B while JSCEM showed only slight activity against B. subtilis, M. fortuitum, S. aureus, S. saprophyticus, S. faecalis, S. pyogenes, E. coli, P. mirabilis, S. paratyphi. B, S. typhi., and S. flexneri. ().
Additionally, three different sizes of seedlings were also extracted with ethyl acetate and methanol. The extracts thus obtained showed weak to moderate antibacterial activity against the microorganisms ().
Antifungal activity
Amongst the extracts of aerial parts and roots, only the P.E. extract (JWP) and (JRP) showed good activity against Candida albicans., having a zone of inhibition (ZOI) of 10 mm. The ethyl acetate extract of first and second type of seedlings, i.e., JSdEAI and JSdEAII, and the methanol extract of first to third type of seedlings, JSdMI, JSdMII, and JSdMIII, were also found to be active against Candida albicans. ().
The flower extract (JFM-EA) showed very strong activity against Trichophyton mentagrophytes. having a ZOI of 24 mm, and it was moderately active against Aspergillus flavus., Fusarium. sp., Penicillium. sp., Rhizopus. sp., and Saccharomyces cerevisiae. with ZOI 10 mm and slightly effective against Aspergillus niger., Candida albicans., and Helementhosporium. with ZOI of 8 mm. One of the VLC fractions, JFMEA-14-15c, was effective against most of the fungi (). The pure compound patuletin (3, JFMEA-13) showed a mild activity against Candida albicans. and Saccharomyces cerevisiae., whereas its derivatives (3a, 3b), patulitrin (4), tetracosanol (5), and all the seed extracts were found to be inactive against all the fungi tested.
Minimum inhibitory concentrations
Minimum inhibitory concentration studies revealed that patuletin (3) was most effective against all Gram-positive bacteria, having a MIC between 12.5 to 100 μ g/disk, whereas it was effective only against few Gram-negative bacteria (MIC 100 μ g/disk). It was followed by 3b (cinnamoylated patuletin), which was effective against almost all Gram-positive and Gram-negative bacteria with a MIC between 50 and > 100 μ g/disk. The activity is in the order JFM-EA 14a-15c > JFM-EA > 3a (). JFM-EA was most effective only against Trichophyton mentagrophytes., and its MIC was found to be 200 μ g/disk.
Conclusions
The above findings suggest that Tagetes patula. is a good source of antimicrobial agents, particularly its flower, which is enriched with flavonoids. One of the easily extractable and nontoxic flavonoids, patuletin (3), exhibited good antibacterial activity, which is comparable with its cinnammoyl derivative (3b). Further investigations on active fractions of flowers and other parts of the plant, as well as SAR studies on patuletin, are under way with a hope that it can provide some promising biologically active constituents that may be used to develop potent antimicrobial drugs.
Acknowledgment
Two of the authors, Samina Bano and Lubna, acknowledge the enabling role of the Higher Education Commission (HEC), Islamabad, Pakistan, and appreciate its financial support through “Award of a research project under national research program for universities (Project# 20-161).”
References
- Abdel-Wahhab M A, Said A, Antje H. NMR and radical scavenging activities of patuletin from Urtica urens. against aflatoxin B. Pharm Biol 2005; 43: 515–525
- Andrews J M, Ashby J P, Wise R. Study to assess the reliability of a disc diffusion method for determining the sensitivity of Gram-positive pathogens to dalfopristin/quinopristin. J Antimicrob Chemothre 1999; 43: 141–143
- Bauer A W, Kirby W MM, Sherris J C, Turk M. Antibiotic susceptibility testing by a standardized single disc method. Am J Clin Pathol 1966; 45: 493–49
- Bhardwaj D K, Bisht M S, Uain S C, Mehta C K, Sharma G C. Quercetagetin 5-methyl ether from the petals of Tagetes patula.. Phytochemistry 1980; 19: 713–714
- Buckingham J. Dictionary of Natural Products, Vol. 5. Chapman & Hall, London 1994; 5367
- Burcher S, Ho W-M. Radical solutions needed for antibiotic resistance. 2001, ISIS Report. Available at http://www.i-sis.org.uk/antibiotic_resistance.php
- Chadha Y R. Tagetes. Linn (Compositae). The Wealth of India, Vol. X. Publications and Information Directorate, CSIR, New Delhi 1976; 109–112
- Chan G FQ, Towers G HN, Mitchell J C. Ultraviolet-mediated antibiotic activity of thiophene compounds of Tagetes.. Phytochemistry 1975; 14: 2295–2296
- Coogan C K, Horn D HS, Lamberton J A. Nuclear magnetic resonance studies. IV. The analysis of the spectra of two thiophene derivatives. Aust J Chem 1965; 18: 723–729
- Copp B R. Antimycobacterial natural products. Nat Prod Rep 2003; 20: 535–557
- Cowan M M. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12: 564–582
- Cushnie T PT, Lamb A J. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005; 26: 343–356
- Faizi S, Dar A, Siddiqi H, Naqvi S, Naz A, Bano S. Bioassay guided isolation of antioxidant agents from flowers of Tagetes patula:. Preparation of new patuletin derivatives. Phytomedicine 2006a, (submitted)
- Faizi S, Naz A. Jafrine, a novel and labile β -carboline alkaloid from the flowers of Tagetes patula.. Tetrahedron 2002; 58: 6185–6197
- Faizi S, Naz A, Siddiqi H, Bano S, Lubna. Specific deuteration in patuletin, and related flavonoids: Solvent and temperature dependent 1H NMR studies. Tetrahedron 2006b, (submitted)
- Gibbons A. Exploring new strategies to fight drug-resistant microbes. Science 1992; 257: 1036–1038
- Gibbons S. Anti-staphylococcal plant natural product. Nat Prod Rep 2004; 21: 263–277
- Ivancheva S, Zdravkova M. Flavonoids in Tagetes patula.. Fitoterapia 1993; 64: 555
- Kagan J. Naturally occurring di- and trithiophenes. Progress in the Chemistry of Organic Natural Products, Vol. 56, W Herz, H Grisebach, G W Kirby, Ch. Tamm. Springer-Verlag/Wien, New York 1991; 87–169
- Kaloshina N A, Mazulin A V. Flavonoids from seeds of Tagetes. Khim Prir Soedin 1983; 1: 104–105
- Kasahara Y, Yasukawa K, Kitanaka S, Khan T M, Evans F J. Effect of methanol extract from flower petals of Tagetes patula. L. on acute and chronic inflammation model. Phytother Res 2002; 16: 217–222
- Kasprzyk Z, Kozierowska T. Distribution of sterols and triterpenic alcohols in plants of Compositae family. Bull Acad Pol Sci Ser Sci Biol 1966; 14: 645–649, (Chem Abstr. 1967, 66, 62617.)
- Koloshina N A, Pasechnik I kh, Zinchenko T V. Flavonoids. U.S. S. R. 1980; 741: 880, (Cl. A61K35/78), 25 July 1980. (Chem Abstr 93., 155840.)
- Kyo M, Miyauchi Y, Fujimots T, Mayama S. Production of nematocidal compounds by hairy root cultures of Tagetes patula.. Plant Cell Report 1990; 9: 393–397
- Lopez-Oviedo E, Aller A L, Martin C, Castro C, Ramirez M, Peman J M, Canton E, Almeida C, Martin-Mazuelos E. Evaluation of disc diffusion method for determining posaconazole susceptibility of filamentous fungi: Comparison with CSLI broth dilution method. Antimicrob Agents Chemother 2006; 50: 1108–1111
- Mares D, Tosi B, Romagnoli C, Poli F. Antifungal activity of Tagetes patula. extracts. Pharm Biol 2002; 40: 400–404
- Margl L, Andreas T, Istvan G, Michael W. GLC and GLCMS Analysis of thiophene derivatives in plants and in vitro. cultures of Tagetes patula. L. Z. Naturforsch C: Biosci. 2002; 57(1/2)63–71
- Mashkovskaja S P, Dzuba O I. Flavonoids of species of Tagetes. L. and their allelopathic activity. Fiziologiya I Biokhimiya Kul‘turnykh Rastenii 2002; 34: 517–523
- Menelaou M A, Fronczek F R, Hjortso M A, Morrison A F, Foroozesh M, Thibodeaux T M, Flores H E, Fischer N H. NMR Spectral data of benzofurans and bithiophenes from hairy root cultures of Tagetes patula. and the molecular structure of isoeuparin. Spectrosc Lett 1991; 24: 1405–1413
- Miyazawa M, Oshima T, Koshio K, Itsuzaki Y, Anzai J. Tyrosinase inhibitor from black rice bran. J Agric Food Chem 2003; 51: 6953–6956
- Narayana K R, Reddy M S, Chaluvadi M R, Krishna D R. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol 2001; 33: 2–16
- Qudrat-e-Khuda. Antibiotic resistance: the challenges ahead. 2003, Available at http://www.dawn.com/weekly/science/archive/030419/science9.htm
- Romagnoli C, Mares D, Fasulo M P, Bruni A. Antifungal effects of α -terthienyl from Tagetes patula. on five dermatophytes. Phytother Res 1994; 8: 332–336
- Saeed A, El-Eraqy W, Ahmed Y. Flavonoids of Urtica urens. L. and biological evaluation. Egypt J Pharm Sci 1995; 36: 415–427
- Sagar D V, Naik S N, Vasudevan P. Antifungal activity of Tagetes patula. essential oil. Indian Perfumer 2002; 46(3)269–272
- Saleem R, Ahmed M, Naz A, Siddiqui H, Ahmad S I, Faizi S. Hypotensive and toxicological study of citric acid and other constituents from Tagetes patula. roots. Arch Pharm Res 2004; 27(10)1037–1042
- Schmeda-Hirschmann G, Tapia A, Theoduloz C, Rodriguez J, López S, Feresin G E. Free radical scavengers and antioxidants from Tagetes mendocina.. Z Naturforsch C J Biosci 2004; 59: 345–353
- Serrano M C, Ramirez M, Morilla D, Chavez M, Espinel-Ingroff A, Fernandez A, Almeida C, Martin-Mazuelos E. A comparative study of the disc diffusion method with the broth microdilution and Etest methods for vorconazole susceptibility testing of Aspergillus. spp. J Antimicrob Chemother 2004; 53: 739–742
- Tarpo E, Cucu V. The flowers of Tagetes. species as raw material for obtaining helenien. Pharmazie 1961; 16: 486–488
- Tereschuk M L, Riera M VQ, Castro G R, Abdala L R. Antimicrobial activity of flavonoids from leaves of Tagetes minuta.. J Ethnopharmacol 1997; 56: 227–232
- Towers G HN, Arnason J T, Wat C K, Lambert J DH. Cercaricidal compositions containing a naturally occurring conjugated polyacetylene and method for controlling cercariae using it. Can. CA 1,169,767, 1984, (Chem Abstr. 101, 146144a.)
- Vasilenko Y K, Bogdanov A N, Frolova L M, Frolov A V. Hepatoprotective properties of preparations from spreading marigold. Khim-Farm Zh 1990; 24(1)53–56
- Vasudevan P, Kashyap S, Sharma S. Tagetes.: A multipurpose plant. Bioresour Technol 1997; 62: 29–35
- Walsh C. Antibiotics: Actions, Origins, Resistance. ASM Press, Washington, DC 2003; 1–328
- Wang Y, Hamburger M, Gueho J, Hostettmann K. Antimicrobial flavonoids from Psiadia trinervia. and their methylated and acetylated derivatives. Phytochemistry 1989; 28(9)2323–2327
- Wells C, Bertsch W, Perich M. Insecticidal volatiles from the Marigold plant (genus Tagetes.). Effect of species and sample manipulatin. Chromatographia 1993; 35(314)209–214
- Xu H-X, Lee S F. Activity of plant flavonoids against antibiotic–resistant bacteria. Phytother Res 2001; 15: 39–43