Abstract
In this work, we studied the effects of the alcohol extract (AE) from Erythrina velutina. Willd (Fabaceae) leaves in animal models of anxiety (elevated plus maze; EPM), memory (inhibitory avoidance test), and epilepsy (pentylenetetrazol-induced convulsions and pentylenetetrazol-induced kindling seizure). In the EPM test, at the dose of 20 mg/kg, the AE significantly increased the percentage of entries into the open arms (p = 0.03). In the inhibitory avoidance test, greater test than training latencies were observed for the control group but not for the AE (10 mg/kg) (p = 0.112) and diazepam (p = 0.331) treated groups during the acquisition phase of the test. During the consolidation and retrieval phases, there were differences between training and test latencies for all three groups. In the pentylenetetrazol-induced convulsion test, the AE (100 mg/kg) increased the latency to death when compared with the control group (p < 0.05) and reduced the kindling behavior induced by low doses of pentylenetetrazol. Together, the effects described for the AE of E. velutina. leaves on rodent central nervous system resemble the profile of effects of benzodiazepines and could be interpreted as an interaction of the extract with GABAergic circuitries.
Introduction
Erythrina velutina. Willd (Fabaceae) is a tree popularly known in Brazil as “mulungu.” In Brazilian folk medicine, the genus Erythrina. is cited for the treatment of central nervous system (CNS) illnesses, especially the species E. velutina. and Erythrine mulung. Mart. The stem bark of the plant is recommended for nervous system excitation, insomnia, convulsions, and nervous coughs (Pio-Corrêa, Citation1984), suggesting that Erythrina. spp. could be useful for the treatment of anxiety and epilepsy.
E. velutina. is the main source of the tetracyclic alkaloids (+)-erythraline and (+)-erythratine, but the flavonoids faseolidine, homoesperatine, erivelutinone, and 3′-O.-methyl-sigmoidine were also isolated from this species (Amer et al., Citation1991).
Some laboratories have recently been testing different extracts from different parts of Erythrina. plants and finding interesting results. For example, Onusic et al. (Citation2002) found that the water-alcohol extract of inflorescences of E. mulungu. was able to reduce anxiety-related responses on the elevated Tmaze and on the light/dark transition model. In a recent publication, we showed that the aqueous extract from leaves of E. velutina. decreased the scores of rats in the open field test and increased sleeping time induced by thiopental (Dantas et al., Citation2004). Ribeiro and colleagues (Citation2006) compared the effects of the water-alcohol extract from the stem bark of E. velutina. and inflorescences from E. mulungu. on avoidance and escape measurements of anxiety on the elevated T-maze test. They concluded that acute and chronic treatment with E. velutina. impaired elevated T-maze avoidance latencies without altering escape, similar to the classic anxiolytic drug diazepam. Together, these data give some scientific support to the popular use of Erythrina. plants as tranquilizers and emphasize the relevance of further investigation on the potential anxiolytic properties of “mulungu.”
Anxiety disorders are among the most prevalent psychiatric diseases in the general population (Somers et al., Citation2006) and are still missing ideal treatments. Today, several groups of drugs are being used for the relief of anxiety symptoms, but they can either produce significant side effects (sedation, cognitive deficit, ataxia, aggression, sexual dysfunction, tolerance, and dependence) or present a delay in the onset of action (Ballenger, Citation1999; Dubovsky, Citation1990; O'Brien, Citation2005). In this context, medicinal plants can be of particular importance, especially in underdeveloped and developing countries, where the WHO recommends their use.
With all that in mind, in the current study, we further explored the potential anxiolytic effects of E. velutina. leaves and also investigated its possible anticonvulsant properties and effects on memory. For that, the alcohol extract of E. velutina. leaves was tested in animal models of anxiety, epilepsy, and memory in comparison with a classic benzodiazepine, diazepam.
Materials and Methods
Plant material
Erythrina velutina. leaves were collected on the metropolitan area of Aracaju, SE, Brazil, during the rainy season (June). The plant was authenticated by Giovani Viana from the Department of Biology at Universidade Federal de Sergipe by comparison with a voucher specimen deposited at the university herbarium (no. ASE 4126). The leaves were dried at 40°C until complete dehydration, and then triturated in a blender until a finely granulated powder was obtained. The alcohol extract (AE) was obtained from this powder, by adding ethanol (final solution: 10%, w/v) and left for 8 days. After filtration in a Buchner funnel, the extract was evaporated in rotavapor, and the solvent residues were removed in high vacuum. Before administration to the animals, the extract was diluted in a solution of distilled water plus cremophor (1:10 v/v).
Animals
Male and female Swiss mice (20–30 g), and male Wistar rats (200–300 g) from our own colony were used. The animals were kept in groups of 10 mice or 5 rats per cage at room temperature and had free access to food and water. The experiments were conducted between 10:00 and 17:00 h. All procedures were in accordance with the UK Animals Scientific Procedures Act 1986.
Treatments
Before the behavioral tests, each animal was treated with either saline (i.p. or p.o.), diazepam (Compaz, Cristália, Brazil; 2, 4, 5, or 10 mg/kg, i.p.), or AE (10, 20, 100, or 500 mg/kg, p.o.). According to these treatments, the groups formed were CTRL, DZP2, DZP4, DZP5, DZP10, Ery10, Ery20, Ery100, and Ery500, respectively. Pentylenetetrazol (PTZ; 90 or 50 mg/kg, i.p.) was also used as convulsant drug in the convulsion models.
Behavioral tests
Elevated plus maze (EPM) test
Experiments were performed in a wooden plus-shaped maze with two closed arms (50 × 10 × 40 cm) and two opens arms (50 × 10 cm), elevated to a height of 50 cm from the floor (Pellow et al., Citation1985). Thirty minutes before being treated with saline (n = 6), diazepam (2 mg/kg, n = 6), AE (10 mg/kg, n = 7), or AE (20 mg/kg, n = 5), rats were individually placed in the center of the maze and allowed to explore the apparatus for 5 min. Time spent in and the number of entries into each type of arm was evaluated. Subsequently, the percentage of time spent in the open arms (%TOA) and the percentage of entries into the open arms (%EOA) were calculated and considered as anxiety parameters. The total distance traveled provided a measure of locomotor activity. All the parameters were measured using a computerized system for animal tracking (Anymaze; Stoelting Co., Wood Dale, IL, USA).
Inhibitory avoidance test
The apparatus consisted of an automated 20 × 50 × 20 cm Perspex box (Hugo Basile, Comerio, VA, Italy) divided by a sliding door into a bright and a dark chamber of the same size. The cage tilting floor consisted of 40 0.3-cm diameter bars of stainless steel, spaced 1.2 cm apart. In the training session, the rats were placed in the bright chamber and the latency to enter into the dark chamber was registered. At the moment they entered, a scramble foot shock of 0.4 mA was applied for 2 s and the animals were immediately removed from the cage. Animals that took more than 60 s to enter the dark compartment were eliminated from the experiment. The test session was held 24 h later, in exactly the same way, except for the foot shock that was now omitted, and the animals were allowed a 180-s latency to enter the dark chamber. The difference between the latencies in the training and test sessions was taken as a measure of retention for the task (Lorenzini et al., Citation1998). Saline (n = 15), diazepam (4 mg/kg, n = 15), or AE (10 mg/kg, n = 15) was administered either 30 min before training, 1 h after training, or 1 h before testing to study their possible effects on memory acquisition, consolidation, and retrieval, respectively (Viana et al., 2001).
Pentylenetetrazol-induced convulsions
One-half hour before being injected with PTZ (90 mg/kg), five groups of 10 mice received saline, AE (20, 100 or 500, mg/kg), or diazepam (5 mg/kg). The latencies to first convulsion and animal death were recorded (Pourgholami et al., Citation1999).
Pentylenetetrazol-induced kindling seizure
For induction of kindling behavior, PTZ (50 mg/kg, i.p.) was injected each 48 h during 10 sessions. One-half hour before each PTZ injection, three groups of 10 rats received either saline or AE (20 and 100 mg/kg). After each injection, animals were monitored by a microcamera during 10 min. The behavior of rats was classified according to the following modified convulsion scale: level 0, no response; level 1, involuntary movements of ears and jaws; level 2, one to 10 myoclonic body jerks; level 3, above 11 myoclonic body jerks; level 4, localized myoclonic convulsion; level 5, generalized convulsion; level 6, death after generalized convulsion (Ilhan et al., Citation2006).
Statistical analysis
The data obtained from the EPM test were analyzed by one-way analysis of variance (ANOVA) followed by Duncan's post hoc test, when appropriate.
Data from the inhibitory avoidance experiment were analyzed by the Wilcoxon signed rank test to compare training-test latency differences and by Mann-Whitney U.-test to compare latency differences between groups.
For the pentylenetetrazol-induced convulsions, data were analyzed by Kruskal-Wallis ANOVA followed, when suitable, by Dunn's post hoc test with CTRL as the reference group.
The different sessions from the kindling experiment were compared through Friedman ANOVA for each treatment group, followed by a nonparametric test for multiple comparisons for data with repeated measures. The comparison among treatment groups in each session was performed by Kruskal-Wallis ANOVA followed by Dunn's post hoc test when adequate.
All significance tests were two-tailed and performed at a 5% significance level.
Results
Elevated plus maze
The ANOVA revealed differences among the groups in total distance traveled [F (3,20) = 3.98; p = 0.02]. As can be seen in , the DZP group traveled a greater distance than all the other groups (CTRL, p = 0.03; Ery10, p = 0.03; Ery20, p = 0.006).
Figure 1 (A) Total distance traveled in the elevated plus maze, and (B) percentage of time in and entries into the open arms of the elevated plus maze of rats treated with saline (CTRL), 10 mg/kg (Ery10), or 20 mg/kg (Ery20) of the alcohol extract of Erythrina velutina. leaves, or 2 mg/kg diazepam (DZP2). Data expressed as mean ± standard error. *Different to CTRL group (p < 0.05); # different to DZP2 group (p < 0.05).
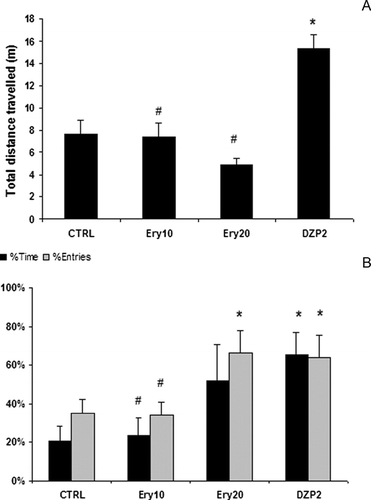
The percentage of time spent in the open arms was also different among groups [F (3,20) = 3.63; p = 0.03] in that the DZP group spent more time in the open arms than did the CTRL (p = 0.02) or AE10 (p = 0.02) groups, and the AE20 group tended (p = 0.09) to spend more time in the open arms, being no different from the saline- but or from the diazepam-treated groups ().
also shows a difference among groups on percentage of entries into the open arms [F (3,20) = 3.81; p = 0.03], in that DZP2 and AE20 groups were significantly different from the control group (p = 0.04 and p = 0.03, respectively).
Inhibitory avoidance
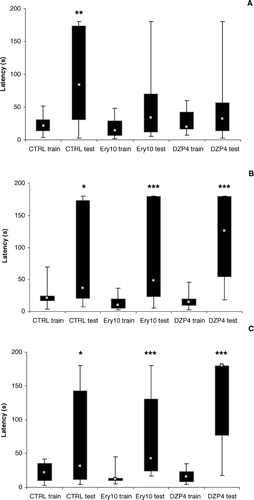
The effects of AE on the behavior of rats in the passive avoidance test are shown in for the acquisition, consolidation, and retrieval phases of memory. The Kruskal-Wallis ANOVA did not show differences among the experimental groups for the entrance into the dark chamber (training session) for the three memory phases (acquisition: H = 2.266, p > 0.05; consolidation: H = 4.997, p > 0.05; retrieval: H = 1.597, p > 0.05). On the other hand, there was a difference between training and test latencies for the CTRL group (T = 4; p = 0.004), but not for the AE10 (T = 32; p = 0.112) and DZP4 groups (T = 37; p = 0.331) during the acquisition phase of the test (). In the consolidation phase (), there was difference among training and test latencies for the three experimental groups (CTRL: T = 21, p = 0.027; AE10: T = 4, p = 0.001; DZP4: T = 0, p = 0.001). The same result was observed for the retrieval phase (CTRL: T = 21, p = 0.027; AE10: T = 6, p = 0.002; DZP4: T = 0, p = 0.001) ().
Figure 2 Latency to enter into the dark chamber of the inhibitory avoidance box of rats treated with saline (CTRL), 10 mg/kg of the alcohol extract of Erythrina velutina. leaves (Ery10), or 4 mg/kg diazepam (DZP4). (A) Animals treated 30 min before training (train): acquisition phase. (B) Animals treated 1 h after training (train): consolidation phase. (C) Animals treated 1 h before testing (test): retrieval phase. Data expressed as median (gray squares), interquartile range (black boxes), and minimum to maximum values (error bars). Different to training session of the same group: *p < 0.05; **p < 0.005; ***p < 0.0005.
Pentylenetetrazole-induced convulsions
Kruskal-Wallis ANOVA showed differences among the treatment groups in the latency for the first convulsion (H = 22.78, p = 0.0001) in that the DZP10 group was different from the CTRL group (p < 0.001, ).
Figure 3 Latencies to (A) first convulsion and (B) death in the test of pentylenetetrazol-induced convulsions, in animals treated with saline (CTRL), 20, 100, or 500 mg/kg of the alcohol extract of Erythrina velutina. leaves (Ery20, Ery100, or Ery500, respectively), or 5 mg/kg diazepam (DZP5). Data expressed as median (gray squares), interquartile range (black boxes), and minimum to maximum values (error bars). Different to CTRL group: *p < 0.05; **p < 0.005.
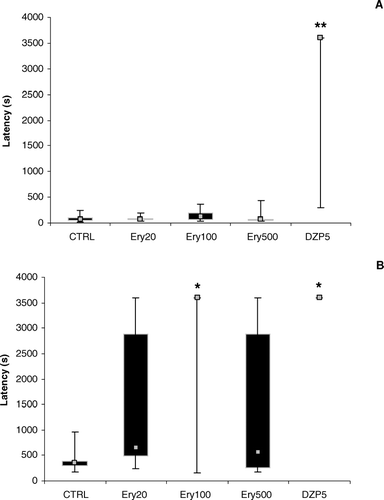
For the latency to death, Kruskall-Wallis ANOVA also showed differences among the treatment groups (H = 17.04, p = 0.0019) in that the DZP5 (p < 0.01) and Ery100 (p < 0.05) groups were different from the CTRL group ().
Pentylenetetrazole-induced kindling seizure
The characteristic kindling behavior, that is, the gradual increase in the intensity of convulsions along the 10 test sessions (S1–S10). was confirmed by Friedman ANOVA (χr2 = 39.33, p = 0.00001) for the CTRL group (). Starting from S3, all the subsequent sessions presented more intense convulsions than S1 (S3: p < 0.00005; S4–S10: p < 0.000005). Also, the convulsions were more intense in S4 than in S3 (p < 0.00005), in S6 than in S5 (p < 0.000005), and in S10 than in S9 (p < 0.000005).
Figure 4 Convulsion scores in the 10 sessions of the pentylenetetrazol induced kindling seizure test. (A) Animals treated with saline. (B) Animals treated with 20 mg/kg of the alcohol extract of Erythrina velutina. leaves. (C) Animals treated with 100 mg/kg of the alcohol extract of Erythrina velutina. leaves. Data expressed as median (gray squares), interquartile range (black boxes), and minimum to maximum values (error bars). Different to S1: *p < 0.05; **p < 0.0005; ***p < 0.000005. Different to the previous session: # p < 0.05; # # p < 0.0005; # # # p < 0.000005.
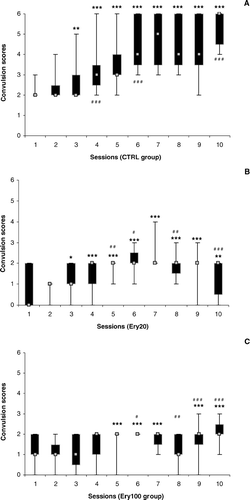
Friedman's test also showed differences among the test sessions for the Ery20 group (χr2 = 22.64, p = 0.007; ). In this case, the intensity of convulsions decreased in S2 and S3 in relation to S1 (p < 0.005 and p < 0.05, respectively). From S4 to S10, the convulsion intensity was greater than in S1 (S4–S9: p < 0.000005; S11: p < 0.0005), but there was not a gradual increase through the sessions. The convulsions were more intense in S5 than in S4 (p < 0.0005) and in S6 than in S5 (p < 0.005), but they were less intense in S8 than in S7 (p < 0.00005), and in S10 than in S9 (p < 0.00005). They never received a score greater than 4.
For the Ery100 group (), differences among test sessions were also found by Friedman ANOVA (χr2 = 18.47, p = 0.03). The first increase in convulsion intensity in relation to S1 appeared only on S5 (p < 0.000005). The convulsions in the subsequent sessions, with exception to S8, were all stronger than in S1 (p < 0.000005), yet there was not a continuing increase in the intensity of convulsions from S5 to S10. The convulsions were more intense in S6 than in S5 (p < 0.05), but they were less intense in S7 than in S6 (p < 0.00005) and in S8 than in S7 (p < 0.00005). Another increase then appeared in S9 in relation to S8 (p < 0.000005) and in S10 in relation to S9 (p < 0.000005). The convulsion scores were never greater than 3.
The comparison among the treatment groups in each test session by Kruskal Wallis ANOVA revealed differences from S1 to S10 (p = 0.0297, p = 0.0013, p = 0.0127, p = 0.0053, p = 0.0011, p = 0.0006, p = 0.0005, p = 0.0009, p = 0.0026, p = 0.0007, respectively). The posttest confirmed the differences only from S2 to S10, with the Ery20 and Ery100 groups presenting less-intense convulsions than did the CTRL group (p < 0.05), with an exception for S3, when Ery20 group missed significance.
Discussion
In this work, we studied the effects of the alcohol extract from Erythrina velutina. leaves in animal models of anxiety, memory, and epilepsy. The results of the EPM test, which evaluates anxiety-like behavior, showed that, at the dose of 20 mg/kg, the AE increased the percentage of entries into the open arms and also tended to increase the percentage of time spent in these arms. These data suggest an acute anxiolytic action of the alcohol extract of E. velutina. leaves. A previous study by Onusic et al. (Citation2002) also found similar results with E. mulungu. (native of southern Brazil). Water-alcohol extract of inflorescences of this plant was able to reduce anxiety related responses on the elevated Tmaze and on the light/dark transition model. More recently, the same group compared the effects of the water-alcohol extract from the stem bark of E. velutina. and inflorescences of E. mulungu. on avoidance and escape measurements of anxiety on the elevated T-maze test. With acute doses of 200 and 400 mg/kg and chronic doses of 50 and 200 mg/kg, they showed impairment of the avoidance responses similar to the reference drug, diazepam (10 mg/kg). Using the EPM model in this work, we demonstrated acute anxiolytic effects of the alcoholic extract from E. velutina. leaves at the dose of 20 mg/kg. Together, these findings are in agreement with the tranquilizing effect known in Brazilian traditional herbal medicine for Erythrina. species (Pio-Corrêa, Citation1984).
In relation to the memory test, 10 mg/kg of AE inhibited the acquisition but not the consolidation and retrieving phases of memory. In a recent publication, we also showed that the aqueous extract of E. velutina. leaves presented this effect at the same dose (Dantas et al., Citation2004). Vianna and colleagues (Citation2001) argued that memory for habituation and inhibitory avoidance tasks are processed by hippocampus, entorhinal cortex, and other brain regions, so these could be the targets for the extract used in the current work. Corroborating this hypothesis, we have shown that the water-alcohol extract from E. velutina. leaves is able to block the excitatory postsynaptic potentials (EPSPs) recorded on rat hippocampal slices (Sarasqueta et al., Citation2002). Currently, a possible interference of this extract with LTP (long term potentiation) is being investigated in a hippocampal slice preparation in our laboratory.
Regarding the anticonvulsant evaluation, acutely the AE at 100 mg/kg was effective in delaying or even preventing death, but it was not able to postpone the start of convulsions. On the other hand, at the same or even in a smaller dose (20 mg/kg), it was able to retard and also minimize the kindling behavior, as the gradual increase in convulsion intensity was replaced by an intensity oscillation, always kept below control values. Therefore, the AE clearly presented an anticonvulsant activity, being more efficient in reducing kindling behavior, induced by systematic application of low doses of PTZ, than preventing acute convulsions induced by a single but higher PTZ dose. In the kindling model, the threshold for convulsions is decreased gradually along the sessions, and a PTZ dose that had minor effects at the beginning of treatment generates a generalized convulsion and death at the end of the 10th PTZ injection. The hippocampal formation is very sensitive to the effects of PTZ and is involved in the reorganization of neuronal circuitry induced by kindling (Morimoto et al., 2004). Therefore, it is possible the AE by modulating neurotransmission in the hippocampus could modulate the generation of seizure induced by PTZ. Although we did not test in this study the effects of diazepam on the generation of kindling behavior, it is well-known that this drug is effective in this model (Hansen et al., Citation2004).
The above-described profile of effects is similar to that of diazepam, a classic benzodiazepine drug, which presents, among other effects, anticonvulsant and sedative properties when given at high doses (> 10 mg/kg), and anxiolytic and amnesic effects at low doses (< 4 mg/kg) (Bennett et al., Citation1990). It is worth emphasizing that, in animals, this amnesic effect only appears when diazepam is administered before the training session in the step down type of the inhibitory avoidance task, similar to what we observed in our avoidance task with E. velutina. extract. It is also worth noticing that E. velutina. leaves present sedative properties as well, as it was observed in our recent study with the aqueous extract, which increased sleeping time duration (10 and 100 mg/kg) and decreased scores in the open field (200 mg/kg) (Dantas et al., Citation2004).
The similar profile of Erythrina. plants to diazepam might be related to their production of alkaloids (Amer et al., Citation1991), which could interact with the GABAergic system as suggested by Garín Aguilar and colleagues (2000). The fact that these and other classes of alkaloids have been isolated from seeds, leaves, stems, bark, roots, pods, and flowers (Garcia-Mateos et al., Citation1981) suggests that these effects can be obtained irrespective of the plant part used. Nevertheless, the use of Erythrina. leaves, as exemplified in our study, seems to be more advantageous in two aspects: first, leaves are more often available than flowers, for example, and plant material availability is important for future development of phytoterapeutic products; second, the collection of leaves does not tend to destroy the plant, as the collection of bark or roots would do.
In summary, though many other preclinical and clinical experiments with Erythrina. spp. still need to be performed in order to establish its therapeutic value, the benzodiazepine-like effects we have observed give some scientific support to the popular use of these plants as tranquilizers and anticonvulsants and supports further research with the isolated alkaloids and flavonoids in the same experimental models.
Acknowledgments
The authors wish to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brasil) for student scholarships for authors D.O.S., M.F.S.A., V.A-N., and I.C.M. de P. (PIBIC/UFS/CNPq) and for a grant for author M. M. (PNEPG, no. 521246/989). This research also received financial support from Fundação de Amparo à Pesquisa e à Inovação Tecnológica de Sergipe (FAPITEC-SE) and Fundação de Apoio à Pesquisa de Sergipe (FAPESE).
References
- Amer M A, Shama M, Freyer A J. The tetracyclic Erythrina. alkaloids. J Natl Prod 1991; 54: 329–363
- Ballenger J C. Current treatments of the anxiety disorders in adults. Biol Psychiatry 1999; 46: 1579–1594
- Bennett D A, Lehmann J, Bernard P S, Liebman J M, Williams M, Wood P L, Boast C A, Hutchison A J. CGS 19755: A novel competitive N-methyl-D-aspartate (NMDA) receptor antagonist with anticonvulsant, anxiolytic and anti-ischemic properties. Prog Clin Biol Res 1990; 361: 519–524
- Dantas M C, De Oliveira F S, Bandeira S M, Batista J S, Silva C D, Jr, Alves P B, Antoniolli A R, Marchioro M. Central nervous system effects of the crude extract of Erythrina velutina. on rodents. J Ethnopharmacol 2004; 94: 129–133
- Dubovsky E V. Generalized anxiety disorder: new concepts and psychopharmacologic therapies. J Clin Psychiatry 1990; 51(Suppl)3–10
- Garcia-Mateos R, Soto-Hernandez M, Kelly D. Alkaloids from six Erythrina. species endemic to México. Biochem Syst Ecol 1981; 26: 545–551
- Hansen S L, Sperling B B, Sanchéz C. Anticonvulsant and antiepileptogenenic effects of GABAA receptor ligands in pentilenetetrazole-kindled mice. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 105–113
- Ilhan A, Iraz M, Kamisli S, Yigitoglu R. Pentylenetetrazol-induced kindling seizure attenuated by Ginkgo biloba. extract (Egb 761) in mice. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1504–1510
- Lorenzini C GA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Temporal characterization of subcortical nuclei in mnemonic processes: results of tetrodotoxin reversible studies in the rat. Arch Ital Biol 1998; 136: 279–296
- O'Brien C P. Bezodiazepine use, abuse and dependence. J Clin Psychiatry 2005; 66: 28–33
- Onusic G M, Nogueira R L, Pereira A MS, Viana M B. Effect of acute treatment with a water alcohol extract from Erythrina mulungu. on anxiety-related responses in rats. Braz J Med Biol Res 2002; 35: 473–477
- Pellow S, Chopin P, File S E, Briley M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neur Meth 1985; 4: 149–167
- Pio-Corrêa M. Dicionário das plantas úteis do Brasil e das exóticas cultivadas, Vol. 5. IBDF, Ministério da Agricultura, Brazil 1984; 262
- Pourgholami M H, Kamalinejad M, Javadi M, Majzoob S, Sayyah M. Evaluation of the anticonvulsant activity of the essential oil of Eugenia caryophyllata. in male mice. J Ethnopharmacol 1999; 64: 167–171
- Ribeiro M D, Onusic G M, Poltronieri S C, Viana M B. Effect of Erythrina velutina Erythrina mulungu. in rats submitted to animal models of anxiety and depression. Braz J Med Biol Res 2006; 39: 263–270
- Sarasqueta D F°, Leite F R, Jr, Araújo-Neto V, Estevam C S, Marchioro M. Efeitos do extrato hidroalcoólico da Erythrina velutina. Willd sobre os potenciais sinápticos em fatias do hipocampo de ratos. FESBE Abstracts 2002; 17: 77
- Somers J M, Goldner E M, Waraichi P, Hsu L. Prevalence and incidence study of anxiety disorders: A systematic review of the literature. Can J Psychiatry 2006; 51: 100–113
- Vianna M RM, Izquierdo L A, Barros D M, de Souza M M, Rodríguez C, Santana M K, Medina J H, Izquierdo I. Pharmacological differences between memory consolidation of habituation to an open field and inhibitory avoidance learning. Braz J Med Biol Res 2001; 34: 233–240
