Abstract
Casearia sylvestris. Sw., also known as “guaçatonga,” is a medicinal plant with broad use in South America. Among the popular applications attributed to this plant are anti-inflammatory, anticancer, antimicrobial, and antiulcer activities. Despite the broad popular use of this plant as a phytopharmaceutical agent, there are few studies about the antimicrobial potential of guaçatonga. In this work, we have studied the antimicrobial potential of an ethanol extract obtained from C. sylvestris. leaves against three yeasts, two filamentous fungus, six Gram-negative bacteria, and two Gram-positive bacteria. Through two chromatographic steps using a Sephadex LH-20 column and RP-HPLC, we isolated and characterized two derived compounds of gallic acid: isobutyl gallate-3,5-dimethyl ether (IGDE) and methyl gallate-3,5-dimethyl ether (MGDE). Both compounds showed antimicrobial activity. IGDE was much more efficient than MGDE in inhibiting yeasts (Candida albicans, Candida tropicalis., and Candida guilliermondii.) and Gram-positive bacteria (Enterococcus faecalis. and Staphylococcus aureus.). This fact is probably associated with the higher hydrophobicity degree of IDGE compared with MGDE.
Introduction
The Flacourtiaceae is a large family consisting of 89 genera and 1300 species found in tropical and temperate regions of the world. In South America, Casearia sylvestris. Sw., popularly named “chá-de-bugre,” “cafezinho-do-mato,” and “guaçatonga,” is a plant used in popular medicine against several diseases (Lorenzi & Matos, Citation2002).
The Brazilian Karajá Indians and Shipibo-Conibo Indians of Peru prepare a bark maceration to treat diarrhea. Other Indian tribes in Brazil mash the roots or seeds of Guaçatonga to treat wounds and topical leprosy. The plant is also a popular herbal medication employed in Bolivian herbal medicine, in which it is considered to be analgesic, antacid, anti-inflammatory, anticancer, and antimicrobial. Nowadays, C. sylvestris. is used as an active principle in pomades against Herpes and is also being commercialized in plant mixtures used in the elaboration of teas and infusions with phytotherapeutic purposes (Alves, Citation2000; Basile et al., Citation1990; Beutler et al., Citation2000; Chiappeta, Citation1983; Mans et al., Citation2000; Menezes et al., Citation2004; Morita et al., Citation1991; Mors et al., Citation2000; Raslan et al., Citation2002; Simões et al., Citation2001).
Concerning the popular use of C. sylvestris., we verified that few studies have been performed at a deeper level regarding its antimicrobial potential. The majority of guaçatonga studies are restricted to its antileishmanial, trypanocidal, larvicidal, gastric antiulcer, anti-inflammatory, genotoxicity, and antioxidant activities (Basile et al., Citation1990; De Mesquita et al., Citation2005; Esteves et al., Citation2005; Maistro et al., Citation2004; Rodrigues et al., Citation2006). However, Mosaddik et al. (Citation2004) showed that the leaves of other genres of Casearia. plants (C. costulata. Jessup., C. grewiifolia. Benth., C. grayi. Jessup, and C. multinervosa. CT White & Sleumer) can inhibit Candida albicans., Escherichia coli., Pseudomonas aeruginosa., and Staphylococcus aureus. growth.
In this work, we studied the antimicrobial potential of ethanol extracts from leaves of C. sylvestris., as we isolated, purified, and characterized two compounds of this material that showed antibacterial and antifungicidal activity.
Materials and Methods
Plant material and alcohol extract
The fresh leaves of C.. sylvestris were collected in the city of Campinas, S.P., Brazil, in March 2005. The material was identified by the botanists from the herbarium of the Biological Institute of Unicamp, where a voucher was deposited under the number UEC 118743. The ethanol extract was produced by using 500 g of fresh leaves that were washed and stirred with 1000 mL of ethanol (analytical grade) in a blender for 15 min at room temperature and then filtered using analytical filter paper. The alcohol extract was centrifuged at 30,000× g. for 15 min, and the supernatant was concentrated to semisolid paste using a rotavapor. This paste was lyophilized to yield 5.6 g of a light-green powder that was stored at −20°C (Weniger, Citation1991).
Sephadex LH-20 column chromatography
A 1.3 g portion of powder obtained from ethanol extract was dissolved in 10 mL of HPLC-grade methanol and applied to a column (1.5-cm diameter and 77-cm height) packed with Sephadex LH-20 (particle size 25–100 mm; Sigma Chemical Co., Nepean, ON, Canada) and eluted with methanol. Methanol fractions (8 mL each) were collected in test tubes placed in an LKB Bromma 2112 Redirac fraction collector (Pharmacia, Uppsala, Sweden) and their absorbances read at 280 nm. Eluates were then pooled into fractions I–VIII. Solvent was evaporated under vacuum at 40°C. Dried fractions were stored in tinted glass bottles at −20°C until used.
Analytical and preparative reverse phase high-performance liquid chromatography
Fractions eluted from Sephadex LH-20 column chromatography were subjected to a second chromatography process using reverse phase high-performance liquid chromatography (RP-HPLC). A Shimadzu HPLC system (Kyoto, Japan) was used for analytical and preparative HPLC of isolated compounds. Conditions for preparative HPLC were as follows: Hilber prepacked column RT (10 × 250 mm) with Lichrosorb RP-18 (Merck, Darmstadt, Germany); water:acetonitrile:methanol:acetic acid (79.5:18:2:0.5, v/v/v/v) as the mobile phase; flow rate of 3 mL/min; UV-Vis spectrophotometric detector adjusted at 280 nm. Each peak eluted was collected, the solvent was evaporated under vacuum at 40°C, and finally was stored at − 20°C until used.
Microorganisms
The microorganisms are characterized and stocked in the microbiology laboratory of the Biology Institute of the State University of Campinas (UNICAMP). The microorganisms were three yeasts (Candida albicans, Candida tropicalis., and Candida guilliermondii.), two filamentous fungi (Aspergillus flavus. and Aspergillus niger.), six Gram-negative bacteria (Escherichia coli., Salmonella enteritidis, Shigella sonnei, Pseudomonas aeruginosa, Serratia marcescens. and Klebsiella pneumoniae.), and two Gram-positive bacteria (Enterococcus faecalis. and Staphylococcus aureus.), obtained from clinical isolates of several materials from the Clinical Hospital of the State University of Campinas (UNICAMP, Campinas, Brazil).
Proton and carbon nuclear magnetic resonance spectroscopy
The compounds selected from RP-HPLC were characterized by proton (1H) and carbon (13C) nuclear magnetic resonance (NMR)s. NMR spectra were recorded using a General Electric 300-NB spectrometer (General Electric, Palo Alto, CA, USA). 1H (at 300 MHz), (1H) correlation spectroscopy (COSY, at 300 MHz), and 13C (at 75.5 MHz) NMR data were collected at room temperature in deuterated methanol (CD3OD). Chemical shifts (δ, ppm) were reported relative to tetramethylsilane (TMS) as an internal standard.
Antimicrobial tests
The microorganisms were grown in appropriately fortifed agar poured in bioassay plates. Mueller-Hinton agar (DIFCO, Detroit, MI, USA) was prepared for the bacteria. RPMI 1640 standard cell culture (Sigma Chemicals, St. Louis, MO, USA) buffered with a 0.165 M MOPS buffer and fortified with 20 g of glucose per liter of 1.5% agar was used for the fungi (Hoffman & Pfaller, Citation2001).
The test solutions were obtained from fractions (or isolated compounds) dissolved in aqueous solution of DMSO (dimethyl sulfoxide) 1% (final concentration of 40 mg/mL). The dilutions for MIC (minimum inhibitory concentration) determinations were serially done (two-fold) up to a concentration of 4.9 μ g/mL. Test solution (100 μ L) was added to the 6.2-mm-diameter wells in agar (Barry, Citation1986), as well as the positive and negative controls. The positive controls were prepared using antibiotics solutions (cloramphenicol and streptomycin for bacteria and miconazol for fungi) in a 1 mg/mL concentration. The solvent was used as the negative control (1% aqueous DMSO).
The activity of fractions from the ethanol extract (and isolated compounds) of C. sylvestris. leaves was determined by inhibition zone (IZ) measurement of fungi or bacteria growth caused by the test solution. The absence of IZs (visual analysis) indicated the last dilution allowing microbial growth, thus it was considered the MIC (NCCLS, Citation1990). Bacteria were added directly to the cooling agar (at about 55°C), and spores for the fungus and yeast were applied as a lawn just prior to coring and filling the wells. Plates were stored for both bacteria and fungi screening for 48 h in an incubator at 37°C. Then, measurements of inhibition zones were done.
Results and Discussion
The results of chromatography using Sephadex LH-20 column are shown in . There were eight peaks with only peak III demonstrating activity against the microorganisms tested. For selection of peaks with antimicrobial activity, only Escherichia coli. and Candida albicans. were used.
Figure 1 Chromatographic profile of elution of ethanol extract from leaves of C. sylvestris. using Sephadex LH-20 column. The peak III showed antimicrobial activity against Escherichia coli. and Candida albicans..
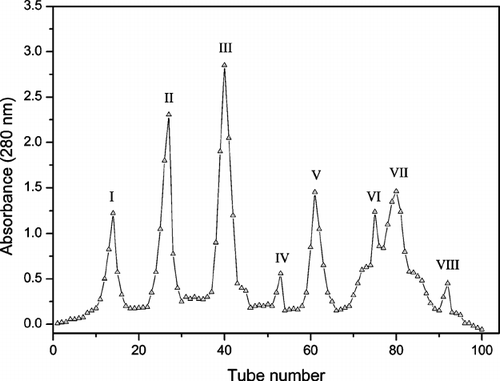
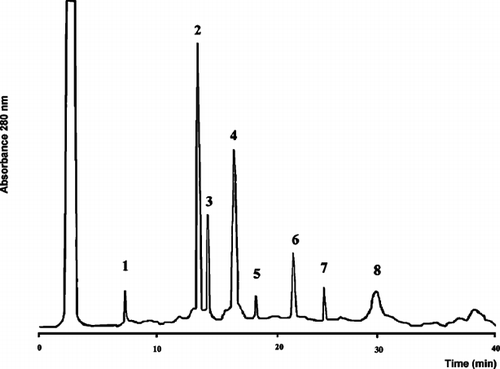
The fractions corresponding with peak III were pooled and lyophilized. This material was dissolved in methanol and subjected to RP-HPLC chromatography. The chromatographic profile obtained is shown in . Peaks 2 and 3 were selected because of antimicrobial activity against Escherichia coli. and Candida albicans., and structures were solved by NMR.
Figure 2 The chromatographic profile of fractions elution, corresponding with peak III (see ) using RP-HPLC. Peaks 2 and 3 showed activity against Escherichia coli. and Candida albicans..
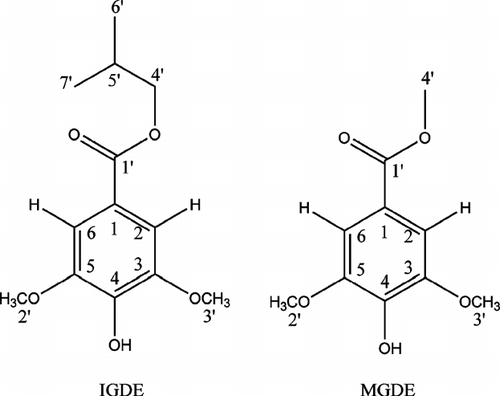
The compounds correspondenting with peaks 2 and 3 were characterized by 1H and 13C NMR spectroscopy. We identified two compounds derived of gallic acid (3,4,5-trihidroxybenzoic acid). The compound corresponding with peak II was identified as isobutyl gallate-3,5-dimethyl ether (IGDE) (), and the compound corresponding to peak III was identified as methyl gallate-3,5-dimethyl ether (MGDE) (). The spectroscopic data a are: as follows: IGDE1H NMR: δ. ppm 7.02 (2H, s, H-2 and H-6), 3.58 (6H, s, H-2′ and H-3′), 2.80 (2H, d, H-4′), 2.20–2.05 (1H, multi, H-5′), 0.98 (6H, s, H-6′ and H-7′); 13C NMR: δ. ppm C 188.02 (C1′), 148.02 (C4), 126.42 (C2 and C6), 124.28 (C3 and C5), 124.02 (C1), 50.01 (C4′), 25.18 (C5′), 22.94 (C6′ and C7′), 19.88 (C2′ and C3′); and MGDE1:H NMR: δ. ppm 6.92 (2H, s, H-2 and H-6), 3.64 (6H, s, H-2′ and H-3′), 2.60 (3H, d, H-4′); 13C NMR: δ. ppm C 186.44 (C1′), 150.61 (C4), 134.28 (C1), 128.28 (C2 and C6), 124.48 (C3 and C5), 30.80 (C4′), 24.28 (C2′ and C3′).
Figure 3 Structures of isobutyl gallate-3,5-dimethyl ether (IGDE) and methyl gallate-3,5-dimethyl ether (MGDE).
The complete antimicrobial assays using either IDGE or MGDE compounds are shown in . Among the microorganisms tested, the Gram-positive bacteria. E. faecalis. and S. aureus. were the most sensitive microorganisms either to IGDE (MIC 9.8 μ g/mL for both) or MGDE (MIC 39.0 μ g/mL for both). The Gram-negative bacteria, E. coli, S. enteritidis., and P. aeruginosa., showed just some degree of sensibility to IGDE and MGDE (see ). The C. albicans, C. tropicalis., and C. guilliermondii. yeasts were significantly sensitive for IGDE (MIC 78.0, 156.0, and 156.0 μ g/mL, respectively) but not to MGDE. No filamentous fungi tested (A. flavus. and A. niger.) was sensitive to IGDE or MGDE.
Table 1 The minimal inhibitory concentrationFootnotea. of IGDE and MGDE (in μ g/mL) against bacteria and fungi.
According to our results, the Gram-positive bacteria and yeasts are more sensitive to the most hydrophobic compound (IGDE) relative to the less hydrophobic compound (MGDE). Similar to our results, Shibata et al. (Citation2005) showed that S. aureus. is sensative to alkyl gallates, and higher hydrophobicity of the tested compound leads to greater inhibition of bacterial growth. Shibata et al. (Citation2005) verified that the presence of alkyl gallates synergistically increases the sensitivity of S. aureus. to β -lactam antibiotics. Also, Kubo et al. (Citation2002) showed that S. cerevisiae. is sensitive to alkyl gallate derivatives with bigger hydrophobicity index.
We have verified that MGDE was in a general way less efficient than IGDE for inhibiting microbial growth. Probably this fact is related to the low degree of hydrophobicity of MGDE, which is chemically much more similar to the gallic acid than IGDE. In fact, Chanwitheesuk et al. (Citation2007) verified that gallic acid inhibits neither C. albicans. nor Aspergillus. spp. growth. Furthermore, these authors verified that Gram-negative bacteria have low sensitivity to gallic acid, but Gram-positive bacteria are very sensitive to the same compound.
Finally, we can conclude that C. sylvestris. presents a good action potential against microorganisms, mainly against Gram-positive bacteria and yeasts. A part of this antimicrobial activity is associated with two compounds derived from gallic acid, IGDE and MGDE.
Acknowledgment
We would like to thank CNPq, CAPES and FAPEAM (Brazilian Agencies) for their financial support.
References
- Alves T MA. Biological screening of Brazilian medicinal plants. Memorial do Instituto Oswaldo Cruz 2000; 95: 367–373
- Barry A. Procedure for testing antimicrobial agents in agar media: Theoretical considerations. Antibiotics in Laboratory Medicine, 2nd ed, V Lorian. Williams & Wilkins, Baltimore 1986; 1–26
- Basile A C, Sertie J A, Panizza S, Oshiro T T, Azzolini C A. Pharmacological assay of Casearia sylvestris.. 1: Preventive anti-ulcer activity and toxicity of the leaf crude extract. J Ethnopharmacol 1990; 30: 185–197
- Beutler J A, Mccall K L, Herbert K, Herald D L, Rettit G R, Johnson T, Shoemaker R H, Boyd M R. Novel cytotoxic diterpenes from Casearia arborea.. J Nat Prod 2000; 63: 657–659
- Chanwitheesuk A, Teerawutgulrag A, Kilburn J D, Rakariyatham N. Antimicrobial gallic acid from Caesalpinia mimosoides. Lamk. Food Chem 2007; 100: 1044–1048
- Chiappeta A D. Higher plants with biological activity plants of Pernambuco. Rev Inst Antibiot 1983; 21: 43–50
- De Mesquita M L, Desrivot J, Bories C, Fournet A, De Paula J E, Grellier P, Espindola L S. Antileishmanial and trypanocidal activity of Brazilian Cerrado plants. Memorias do Instituto Oswaldo Cruz 2005; 100: 783–787
- Esteves I, Souza I R, Rodrigues M, Cardoso L GV, Santos L S, Sertie J AA, Perazzo F F, Lima L M, Schneedorf J M, Bastos J K, Carvalho J CT. Gastric antiulcer and anti-inflammatory activities of the essential oil from Casearia sylvestris. Sw. J Ethnopharmacol 2005; 101: 191–196
- Hoffman H L, Pfaller M A. In vitro. antifungal susceptibility testing. Pharmacotherapy 2001; 21: 111–123
- Kubo I, Xiao P, Nihei K-I, Fujita K-I, Yamagiwa Y, Kamikawa T. Molecular design of antifungal agents. J Agric Food Chem 2002; 50: 3992–3998
- Plantas Medicinais no Brasil: Nativas e exóticas, H Lorenzi, F JA Matos. Inst. Plantarum Est. Flora LTDA, Nova Odessa 2002; 23–24
- Maistro E L, Carvalho J CT, Mantovani M S. Evaluation of the genotoxic potential of the Casearia sylvestris. extract on HTC and V79 cells by the comet assay. Toxicol Vitro 2004; 18: 337–342
- Mans D RA, Da Rocha A B, Schwartsmann G. Anti-cancer drug discovery and development in Brazil: Targeted plant collection as a rational strategy to acquire candidate anti-cancer compounds. Oncologist 2000; 2000: 185–198
- Menezes P R, Schwarz E A, Santos C AM. In vitro. antioxidant activity of species collected in Paraná. Fitoterapia 2004; 75: 398–400
- Morita H, Nakayama M, Kojima H, Takeya K, Itokawa H, Schenkel E P, Motidom M. Structure and cytotoxic activity relationship of casearins, new clerodane diterpenes from Casearia sylvestris. SW. Chem Pharm Bull 1991; 39: 693–697
- Mors W B, Nascimento M C, Pereira B MR, Pereira N A. Plant natural products active against snake bite - the molecular approach. Phytochemistry 2000; 55: 627–642
- Mosaddik M A, Banbury L, Foster P, Booth R, Markham J, Leach D, Waterman P G. Screening of some Australian Flacourtiaceae species for in vitro. antioxidante, cytotoxic and antimicrobial activity. Phytomedicine 2004; 11: 461–466
- NCCLS (National Commitee for Clinical Laboratory Standards). Standard methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS, Villanova, PA 1990; 143–152, Approved standard M7-45
- Raslan D S, Jamal C M, Duarte D S, Borges M H, De Lima M E. Anti-PLA2 action test of Casearia sylvestris. Sw. Boll Chim Farm 2002; 141: 457–460
- Rodrigues A MS, De Paula J E, Degallier N, Molez J F, Espi'ndola L S. Larvicidal activity of some Cerrado plant extracts against Aedes aegypti.. J Am Mosq Cont Ass 2006; 22: 314–317
- Shibata H, Kondo K, Katsuyama R, Kawazoe K, Sato Y, Murakami K, Takaishi Y, Arakaki N, Higuti T. Alkyl gallates, intensifiers of β -lactam susceptibility in methicillin-resistant Staphylococcus aureus.. Antimicrob Agents Chemother 2005; 49: 549–555
- Farmacognosia da planta ao medicamento, C MO Simões, E P Shenkel, G Gosman, J CP Mello, L A Mentz, P R Petrovick. UFRGS/UFSC, Florianópolis 2001; 14–39
- Weniger B. Theory and instrumentation involved with extraction, control, quality insurance and registration of natural products. First International Advanced Course on Technology and Control of Drugs, Italy, Perugia, 1991; 31–40
