Abstract
Xylopia championii. Hook. f. & Thoms. (Annonaceae) is endemic to Sri Lanka. The antioxidant and antifungal activities of five alkaloids, oxopurpureine, (+)-laudanidine, (−)-discretine, nordicentrine, and dehydrocorytenchine, isolated from the stem bark and stem of X. championii., were studied. The alkaloids, (+)-laudanidine and (−)-discretine, at a concentration of 0.5 mg/mL, exhibited exceptionally high antioxidant activity, where as nordicentrine and dehydrocorytenchine showed moderate activity as compared with the standard antioxidant dl-α -tocopherol in the DPPH assay. All five alkaloids were subjected to an antifungal bioassay against Clodosporium cladosporioides.. Nordicentrine showed the most potent antifungal activity at 6 μ g/spot, and (-)-discretine showed moderate activity at 30.0 μg/spot.
Introduction
The family Annonaceae is important phytochemically because of the frequent presence of isoquinoline alkaloids and, more recently, on the basis of the restrictive occurence of a very active class of natural products, the acetogenins (Chang et al., Citation1998). It comprises 130 genera and some 2300 species. The genus Xylopia. (Annonaceae) has been described in several parts of the world, mainly in tropical regions of South and Central America, Africa, and Asia. Plants of the genus Xylopia. have yielded products of different classes, such as acetogenins, alkaloids, amides, flavonoids, lignoids, and terpenoids (Moreira et al., Citation2005). The various extracts from Xylopia. spp. have been shown to possess antiseptic and analgesic properties and insecticidal activity against adult mosquitoes, several leaf-eating insects, and houseflies (Stashenko et al., Citation2004). The essential oil of Xylopia aethiopica. (Dunal) A. Rich fruits was active against fungi and was also lethal to brine shrimps and Hep-2 carcinoma cell lines (Asekun & Adeniyi, Citation2004).
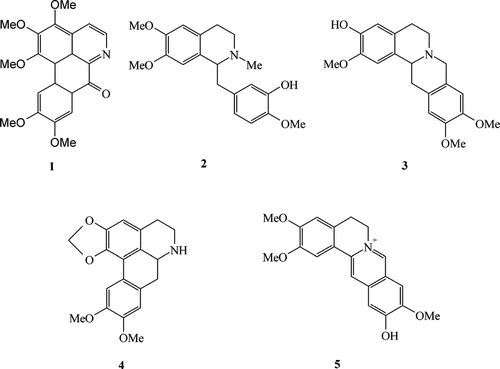
Three species of Xylopia. are native to Sri Lanka, two of which are endemic. One of them, Xylopia championii. Hook. f. & Thoms., is a common understorey tree of the humid lowland endemic to the southwestern part of Sri Lanka (Dissanayake & Fosberg, Citation1985). A previous report described the isolation of two oxoaporphine alkaloids, O.-methylmoschatoline and dicentrinone, from the stem bark of X. championii. (Wijeratne et al., Citation1996). As part of a study aimed at the isolation of biologically active compounds from Annonaceae species endemic to Sri Lanka, the isolation and antifungal and antioxidant assessment of five other known alkaloids, namely, oxopurpureine (1), (+)-laudanidine (2), (−)-discretine (3), nordicentrine (4), and dehydrocorytenchine (5) () from the stem bark and stem of X. championii. are reported herein.
Materials and Methods
General experimental procedures
Melting points (uncorrected) were determined by using a Kofler hot-stage apparatus (A. H. Thomas Company, Philadelphia, PA). UV absorptions were measured with a Shimadzu 1601 UV spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1H and 13C NMR, correlation spectroscopy (COSY), distortionless enhancement by polarization transfer (DEPT), heteronuclear correlated spectroscopy (HETCOR), heteronuclear multiple quantum correlation (HMQC), heteronuclear multiple bond correlation (HMBC), and nuclear Overhauser spectroscopy (NOESY) (Varian Inc., Palo Alto, CA, USA) spectra were recorded on a Varian (1H 300 and 13C 75.45 MHz) NMR spectrophotometer in CDCl3 with TMS as the internal standard. Low- and high-resolution electron impact mass spectra were recorded on a Kratos/AEI MS-902 spectrometer (Manchester, UK). Silica gel used was Merck Kieselgel (230–400 mesh ASTM) (Darmstadt, Germany).
Plant collection
X. championii. was collected from the Kitulgala Forest, Central Sri Lanka, in December 2004 by Dr. D.S.A. Wijesundara. A voucher specimen has been deposited at the National Herbarium, Royal Botanic Gardens (Peradeniya, Sri Lanka).
Extraction and isolation of alkaloids
Air-dried stem bark and stem of X. championii. (6 kg) was ground into a powder and sequentially extracted into CH2Cl2 and MeOH (8 L each) using a bottle shaker at room temperature. Solvent was removed under reduced pressure (< 35°C) using a rotavapor. The CH2Cl2 extract (17 g) was dissolved in CHCl3 and was partitioned with 2 N HCl. The aqueous layer was basified with 20% NH4OH and partitioned again with CHCl3. The organic layer was separated and evaporated under reduced pressure (< 35°C) to yield a brown-colored crude alkaloid mixture (4 g). The crude mixture was subjected to MPLC (Medium Pressure Liquid Chromatography) (eluent: hexane to CH2Cl2 to 20% MeOH/CH2Cl2) yielding three fractions that were Dragendorff positive. Gravity column chromatography on silica gel (eluent: hexane to EtOAc to 10% MeOH/EtOAc) yielded the following compounds:
Oxopurpureine. (1, 14 mg): Dark-orange needles (CH2Cl2), m.p. 199–201°C; EIMS m./z. 382 [M]+ (Sonnet & Jacobson, Citation1971; Chang et al., Citation1998).
(+)-Laudanidine. (2, 7 mg): Brown-colored powder, m.p. 178–180 °C; [α]D.27.4 = +150° (c. 0.0001, CH2Cl2); EIMS m./z. 343 [M]+ (Blanchfield et al., Citation2003).
An alkaloid wash, as described above, was also carried out for the methanol extract (57 g) to yield 5 g of a crude alkaloid mixture. This crude mixture when subjected to MPLC (Medium Pressure Liquid Chromatography) (eluent: hexane to CH2Cl2 to MeOH) followed by flash chromatography (eluent: 20% CH2Cl2/hexane to CH2Cl2 to 7.5% MeOH/CH2Cl2) and gravity column chromatography (eluent: hexane to CH2Cl2 to 30% MeOH/CH2Cl2) yielded the following:
(-)-Discretine. (3, 45 mg): sticky solid, [α]D.27.4 = −300° (c. 0.0002, CH2Cl2); EIMS m./z. 342 [M]+ (Hocquemillar et al., Citation1984).
Nordicentrine. (4, 41 mg): sticky solid, EIMS m./z. 326 [M]+ (Likhiwitayawuid et al., 1993).
Dehydrocorytenchine. (5, 80 mg): green crystals (CH2Cl2), 260–262°C; EIMS m./z. 339 [M]+ (Jossang et al., Citation1991).
Antifungal assay: TLC bio-autography
Serial dilutions of samples of known concentrations were spotted (1 μ L/spot) on a TLC plate for minimum inhibitory dose (MID) determination and were sprayed adequately with a spore suspension of the fungus Clodosporium clodosporioides. in Czapec-dox nutrient solution. The sprayed TLC plates were incubated in a wet chamber at 25 ± 2°C for 48 h in the dark. Antifungal compounds appeared as clear inhibition zones (white zones) against the dark background of C. clodosporioides. spores. The lowest dose (μ g/spot) of sample to show an inhibition zone was recorded as the MID (Homans & Fuchs, Citation1970; Mackeen et al., Citation2002).
DPPH antioxidant assay
A methanol solution of DPPH (1 × 10− 4 M) was prepared and stored at 10°C in the dark. A methanol solution of the test compound was prepared (0.5 mg/mL). A 40-μ L aliquot of the methanol solution was added to 3 mL of DPPH solution. Absorbance measurements were recorded immediately with a UV-visible spectrophotometer. The decrease in absorbance at 515 nm was determined continuously, with data being recorded at 1-min intervals until the absorbance stabilized (16 min). The absorbance of the DPPH radical without antioxidant and the reference compound dl-α -tocopherol also was measured. All the determinations were performed in three replicates and averaged. The percentage inhibition of the DPPH radical was calculated according to the formula.
where Ac = absorbance of the control at t. = 0 min, and Aa = absorbance of the DPPH at t. = 16 min (Yen & Duh, Citation1994).
Results and Discussion
The scavenging activity of the alkaloids isolated from X. championii. against the DPPH radical is shown in . The alkaloids, (+)-laudanidine (2) and (−)-discretine (3), at a concentration of 0.5 mg/mL, exhibited exceptionally high antioxidant activity compared with dl-α -tocopherol, the positive control. Significantly, for (+)-laudanidine (2) and (−)-discretine (3), there are no previous reports of biological activity. Alkaloids nordicentrine (4) and dehydrocorytenchine (5) showed moderate activity in the DPPH assay.
Table 1 Antifungal activity and scavenging effect of the alkaloids 1– 5 of X. championii. on 1,1-diphenyl-2-picrylhydrazyl radical.
Preliminary investigation of the crude alkaloid extracts from dichloromethane and methanol extracts of the stem bark and stem of X. championii. showed a positive response in an antifungal bioassay against C. cladosporioides. using the TLC bio-autography method (Homans & Fuchs, Citation1970). Subsequently, the pure alkaloids 1– 5 were subjected to the antifungal bioassay. Nordicentrine (4) showed the most potent antifungal activity at 6 μ g/spot, whereas (−)-discretine (3) showed moderate activity at 30.0 μ g/spot.
The alkaloid oxopurpureine (1), previously isolated from Annona purpurea L.., effected the aggregation of rabbit platelets induced by thrombin, arachidonic acid, collagen, and platelet activating factor (Chang et al., Citation1998) and also showed borderline activity in vitro. against the KB cell culture test system (Sonnet & Jacobson, Citation1971). Nordicentrine (4) showed general cytotoxicity in several cancer cell lines (Stévigny et al., Citation2005). It also showed antimalarial activity and cytotoxicity to cancer cell lines (Likhitwitayawuid et al., Citation1993). O.-Methylmoschatoline, isolated from the bark of Cananga odorata. Hook. f. & Thomas (also found in X. championii.), showed antibacterial activities against a number of Gram-positive and Gram-negative bacteria. This compound also exhibited antifungal (at the concentration of 400 μ g/disk) and cytotoxic activities (Rahman et al., Citation2005), and antileishmanial activity against promastigote forms of Leishmania braziliensis. (Costa et al., Citation2006). However, O.-methylmoschatoline, isolated by us from X. championii., showed very low antioxidant activity and it did not inhibit the growth of the fungus C. cladosporioides..
Acknowledgments
The authors thank the National Science Foundation, Sri Lanka, for generous funding, the National Herbarium, Peradeniya Botanic Gardens, for identifying the plant material, and the Department of Botany, University of Peradeniya (Peradeniya, Sri Lanka) for providing facilities to carry out the antifungal assay.
References
- Asekun O T, Adeniyi B A. Antimicrobial and cytotoxic activities of the fruit essential oil of Xylopia aethiopica. from Nigeria. Fitoterapia 2004; 75: 368–370
- Blanchfield J T, Sands D PA, Kennard C HL, Byriel K A, Kitching W. Characterization of alkaloids from some Australian Stephania. (Menispermaceae) species. Phytochemistry 2003; 63: 711–720
- Chang F R, Wei J L, Teng C M, Wu Y C. Antiplatelet aggregation constituents from Annona purpurea.. J Nat Prod 1998; 61: 1457–1461
- Costa E V, Pinheiro M BP, Xavier C M, Silva J RA, Amaral A CF, Souza A DL, Barison A, Campos F R, Ferreira A G, Machado G MC, Leon L LP. A primidine- β-carboline and other alkaloids from Annona foetida. with antileishmanial activity. J Nat Prod 2006; 69: 292–294
- Dissanayake M D, Fosberg F R. A Revised Handbook to the Flora of Ceylon, Vol. V. Amernd Publishing Co. Pvt Ltd., New DelhiIndia 1985; 1–75
- Hocquemillar R, Debitus C, Roblot F, Cavé A. Alcaloïdes des annonacées, XLVIII. Alcaloïdes des écorces de Guatteria discolor.. J Nat Prod 1984; 47: 353–362
- Homans A L, Fuchs A. Direct bioautography on thin layer chromatograms as a method for detecting fungitoxic substances. J Chromatogr 1970; 51: 327–329
- Jossang A, Lebceuf M, Cave A, Pusset J. Alcaloïdes des Annonacées, 91. Déhydroxylopine et déhydrocorytenchine, nouveaux alcaloïdes isoquinoléiques isolés de Xylopia vieillardi.. J Nat Prod 1991; 54: 466–472
- Likhitwitayawuid K, Angerhofer C K, Chai H, Pezzuto J M, Cordell G A, Ruangrungsi N. Cytotoxic and antimalarial alkaloids from the tubers of Stephania pierrei.. J Nat Prod 1993; 56: 1468–1478
- Mackeen M MA, Ali M, Lajis N H, Kawazuc K, Kikuzaki H, Nakatani N. Antifungal garcinia acid esters from the fruits of Garcinia atroviridis.. Z Naturforsch 2002; 57c: 291–295
- Moreira I C, Lago J OHG, Roque N DF. Sesquiterpenes, diterpenes, steroids and alkaloids from branches of Xylopia brasiliensis. Spreng (Annonaceae). Biochem Syst Ecol 2005; 33: 948–951
- Rahman M M, Lopa S S, Sadik G, Harun-Or-Rashid, Islam R, Khondkar P, Alam A H, Rashid M A. Antibacterial and cytotoxic compounds from the bark of Cananga odorata.. Fitoterapia 2005; 76: 758–761
- Sonnet P E, Jacobson M. Tumor inhibitors II. Cytotoxic alkaloids from Annona purpurea.. J Pharm Sci 1971; 60: 1254–1256
- Stashenko E E, Jaramillo B E, Martinez J R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica.(Lamarck) by different extraction and headspace methods and gas chromatography. J Chromatogr A 2004; 1025: 105–113
- Stévigny C, Bailly C, Leclercq J Q. Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr Med Chem 2005; 5: 173–182
- Wijeratne E MK, Hatanaka Y, Kikuchi T, Tezuka Y, Gunatilaka A AL. A dioxoaporphine and other alkaloids of two annonaceous plants of Sri Lanka. Phytochemistry 1996; 42: 1703–1706
- Yen G C, Duh P D. Scavenging effect of methanolic extracts of peanut hulls on free- radical and active-oxygen species. J Agric Food Chem 1994; 42: 629–632