Abstract
A salicylic acid derivative (1), a cinnamaldehyde (2), and six isoflavones (3–8) from the stem bark of Flemingia paniculata. Wall. (Leguminosae) were tested for antibacterial (both Gram-positive and Gram-negative) and antifungal activities. All the compounds showed significant activities against the test organisms having MIC values in the range of 1.57–200 μ g/mL. The highest potency (MIC = 1.57 μ g/mL) was exhibited by 3 against Staphylococcus aureus..
Introduction
A number of antibiotics and chemotherapeutic agents of natural or semisynthetic origins are available today to fight against infections caused by various pathogenic microorganisms (e.g., bacteria, fungi, and viruses). However, with the alarming increase of incidences of microbial resistance against many of these antimicrobial drugs, the need for newer, safer, and more effective antimicrobial drugs has become paramount. Microorganisms develop resistance against antimicrobial agents mainly by modification of cell targets rendering insensitivity to antibiotics and/or by inactivation of antimicrobial agents by the action of microbial enzymes. In third World countries, like Bangladesh, self-medication and irrational use of antibiotics may also lead to the development of microbial resistance. As plants are known to produce antimicrobial agents as their defense mechanism, they can be potential sources of new antibacterial agents.
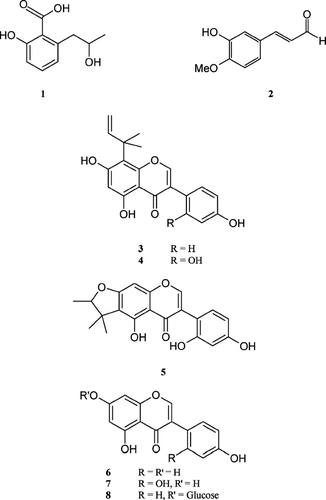
Flemingia paniculata. Wall. (Leguminosae) is an erect branched shrub, 4–6 feet high, with finely downy terete branches. It is distributed widely in Bangladesh, India, Nepal, and other tropical countries (Hooker, Citation1879). The members of the genus Flemingia. are used traditionally in epilepsy, hysteria, to induce sleep and thereby relieve pain, and also as vermifuge for children (Kirtikar et al., Citation1993; Yusuf et al., Citation1994). Previous phytochemical investigation on the genus Flemingia. revealed the presence of chalcones (Subrahmanyam et al., Citation1982), isoflavonoids (Rao & Srimannarayan, Citation1984; Chen et al., Citation1991), and flavanone (Rao & Srimannarayan, Citation1983) and flavonol glycosides (Rao et al., Citation1983). As a part of our continuing phytochemical and bioactivity studies on Bangladeshi medicinal plants (Anjum et al., Citation2002; Haque et al., 2004; Rahman & Gray, Citation2002, Citation2005; Sadik et al., 2003), we now report the antimicrobial activities of a salicylic acid derivative (1), a cinnamaldehyde (2), = and six isoflavones (3–8) () from the stem bark of F.. paniculata. against both Gram-positive and Gram-negative bacteria and fungi.
Materials and Methods
Plant material
The stem bark of F. paniculata. was collected from the Modhupur Forest (Tangail, Bangladesh) in March 2000. The plant was identified by Dr. Toby Pennington, Royal Botanical Garden (Edinburgh, Scotland, UK) where a voucher specimen (MMR-004/RBGE) of this collection has been deposited.
Extraction
The sun-dried and ground plant materials (250 g) were Soxhlet extracted successively with petroleum ether (60–80°C), chloroform, and methanol. The extracts were concentrated with a rotary evaporator at 40–50°C and reduced pressure to obtain the dried extracts. The yields of the extracts were 0.44%, 0.68%, and 0.98%, respectively.
Isolation of constituents
Vacuum liquid chromatographic (VLC) fractionation of the petroleum ether extract (1.5 g) on Si gel using the mobile phase petroleum ether, EtOAc, and MeOH in order of increasing polarity followed by preparative TLC (15% EtOAc in petroleum ether) on the fraction eluting with 10% EtOAc in petroleum ether yielded 3.2 mg of the salicylic acid derivative, 2-carboxy-3-(2-hydroxypropanyl)-phenol (1) (Rahman et al., Citation2004). Similar fractionation of the CHCl3 extract (3.8 g) followed by Sephadex column chromatography and preparative TLC and/or HPLC gave more of 1 (14 mg), 3-hydroxy-4-methoxycinnamaldehyde (2, 3.4 mg) (Barakat et al., Citation1987), 5,7,4′ -trihydroxy-8-(1,1-dimethylprop-2-enyl)-isoflavone (3, 5 mg) (Rahman et al., Citation2004), 5,7,2′,4′ -tetrahydroxy-8-(1,1-dimethylprop-2-enyl)-isoflavone (4, 11.4 mg) (Rahman et al., Citation2004), and 5,2′,4′ -trihydroxy-4″,4″,5″(ξ)-trimethyl-4″,5″-dihydrofurano-(7,6,2″,3″)-isoflavone (5, 4 mg, Rahman et al., Citation2004). Sep-Pak fractionation of the methanol extract (1.5 g) followed by preparative HPLC afforded genistein (6, 5.2 mg) (Hosny & Rosazza, 1999), 2′ -hydroxygenistein (7, 35 mg) (Krishnamurty & Prasad, 1980), and genistein 7-O-α-d-glucoside (8, 3.2 mg) (Hosny & Rosazza, Citation1999).
Preparation of the test materials and standards
The compounds (1– 8) and antibiotics (amoxicillin and fluconazole) were dissolved in DMSO followed by dilution with peptone water to obtain a concentration of 200 or 400 μ g/mL.
Microorganisms
Both Gram-positive and Gram-negative bacteria and two fungi were used in this study. The list of organisms is given in .
Table 1 Antibacterial and antifungal activities of the constituents of Flemingia paniculata..
Antimicrobial study
The antimicrobial assay was performed by a microdilution titer technique (Sarker et al., Citation2007) using 96-well plates, which offers the advantage of determining the minimum inhibitory concentration (MIC) at the same time. In this test, 100 μ g/mL of the indicator solution (resazurin, 750 μ g/mL) was first placed into the sterility control wells (11th column) on the 96-well plates. About 7.5 mL of indicator solution was then mixed with 5 mL test organism (108 cfu/mL) followed by transferring (100 μ L each) into growth control wells (12th column) and all test wells (1st to 10th columns) on the plates. Sample solutions (100 μ l each) were then applied to the first column of the plates. In a plate, up to six samples could be applied leaving two for negative and positive controls. Once all samples and controls were applied to the first column of wells on the plate, half of the content (100 μ L) from these wells was then transferred to the second column of wells, and each subsequent well was treated similarly (doubling dilution) up to the 10th column, followed by discarding the last 100 μ L aliquot. Finally, the plates were incubated at 37°C for around 5–6 h, until growth control wells developed the growth (pink color). In the case of the fungi, the incubation period was around 12–16 h. The activity was marked by observing the change of color from pink to blue. As the process operates on a doubling dilution of test materials, the lowest concentration at which change of color occurred was considered as the minimum inhibitory concentration (MIC) of a test compound.
Results and Discussion
The antibacterial and antifungal activities of the compounds 1– 8, isolated from the stem bark of Flemingia paniculata., are presented in . All compounds showed significant activities against test organisms except 2 against Klebsiella aerogenes.. 2-Carboxy-3-(2-hydroxypropanyl)-phenol (1), having structural similarity (2-hydroxypropanyl side chain on ortho. position) with salicylic acid, showed activities against all test organisms with MICs in the range 50–200 μ g/mL. Among the compounds, the isoflavones 3– 5 exhibited more potent antimicrobial activities compared with the other compounds. 5,7,4′ -Trihydroxy-8-(1,1-dimethylprop-2-enyl)-isoflavone (3) showed prominent activity against Staphylococcus aureus. with a MIC value of 1.57 μ g/mL (0.005 μ mol). Its activity was counted as half of amoxi-cillin (MIC = 0.89 μ g/mL; 0.0024 μ mol). The order of activities of the compounds in molar concentration against Staphylococcus aureus. was 3 > 4 = 5 > 8 > 2 > 7 > 6 > 1. In terms of molar concentration, compound 3 was 80-times more active against S.. aureus. than genistein (6), the parent compound. Thus its high potency was presumed to be due to the presence of the 1,1-dimethylprop-2-enyl substituent. Genistein (6) and its 7-glucoside (8) showed almost same activities. The presence of glucose at C-7 did not make any appreciable change in activity. The sequences of molar activities of the compounds against E.. coli. and C.. albicans. were 8 > 4 = 5 > 3 > 2 > 7 > 6 > 1 and 4 = 5 > 3 > 2 > 7 > 6 > 8 > 1, respectively. Against C.. albicans., compounds 4 and 5 were the most potent of the test compounds, and compounds showed virtually the same activities against test organisms (except for Proteus vulgaris.). The molecular formulae of these two compounds are the same, and they are very closely related biosynthetically (Rahman et al., Citation2004).
Flavonoids are known to exhibit a range of activities including anti-inflammatory, antithrombotic, antiviral, and hepatoprotective which may, in some measure, be due to their ability to scavenge free radicals (Akdemir et al., Citation2001; Saija et al., Citation1995). Genistein and 2′-hydroxygenistein were reported to be potent inhibitors of indole-3-acetic acid oxidase activity (Ferrer et al., Citation1992). Genistein has also been reported to have strong lipid peroxidation inhibitory effects and cytotoxicity (Cos et al., Citation2001). When tested against oral microorganisms, genistein's MIC was found to be 12.5 μ g/ml against Porphyrmonas gingivalis. and more than 50 μ g/mL against Lactobacillus casei., L.. fermentum., Streptococcus mutans., Fusobacterium nucleatum., Prevotella intermedia., Actinobacillus actinomycetemicomitans., A.. naeslandiim., and Staphylococcus aureus. (Iinuma et al., Citation1994). Thus, the current findings on the antimicrobial activities of the compounds isolated from F. paniculata. have further strengthened the previous findings of effectiveness of certain flavonoids against microbial infections.
Acknowledgment
M.M.R. is grateful to the Association of Commonwealth Universities (ACU), UK, for the award of commonwealth scholarship (BDCA-1999-42).
References
- Akdemir Z S, Tatli I I, Saracoglu I, Ismailoglu U B, Sahin-Erdemli I, Calis I. Polyphenolic compounds from Geranium pratense. and their free radical scavenging activities. Phytochemistry 2001; 56: 189–193
- Anjum A, Haque M E, Rahman M M, Sarker S D. Antibacterial activities of the compounds from the flower of Alangium salviifolium.. Fitoterapia 2002; 73: 526–528
- Barakat H H, Nawaar M AM, Buddrus J, Linscheid M. Niloticol, a phenolic glyceride and two phenolic aldehydes from the roots of Tamarix nilotica.. Phytochemistry 1987; 26: 1837–1838
- Chen M, Lou S Q, Chen J H. 2 Isoflavones from Flemingia philippinensis.. Phytochemistry 1991; 30: 3842–3844
- Cos P, Calomme M, Sindambiwe J B, De Bruyne T, Cimanga K, Pieters L, Vlietinck A J, Berghe V D. Cytotoxicity and lipid peroxidation-inhibiting activity of flavonoids. Planta Med 2001; 67: 515–519
- Ferrer M A, Pedreño M A, Muñoz R, Barceló A R. Constitutive isoflavones as modulators of indole-3-acetic acid oxidase activity of acidic cell wall isoperoxidases from lupin hypocotyls. Phytochemistry 1992; 31: 3681–3684
- Hooker J D. The Flora of British India. L. Reeve & Co., London 1879; 133–134
- Hosny M, Rosazza J PN. Novel isoflavone, cinnamic acid, and triterpenoid glycosides in soybean molasses. J Nat Prod 1999; 62: 1609–612
- Iinuma M, Tsuchiya H, Sato M, Yokoyama J, Ohyama M, Ohkawa Y, Tanaka T, Fujiwara S, Fujii T. Flavanones with potent antibacterial activity against methicillin resistant Staphylococcus aureus.. J Pharm Pharmacol 1994; 46: 892–895
- Kirtikar K R, Basu B D, An I CS. Indian Medicinal Plants. Bishen Singh Mahendra Pal Sigh, India 1993; 121–123
- Krishnamurty H G, Prasad J S. Isoflavones of Moghania macrophylla.. Phytochemistry 1980; 19: 2797–2798
- Rahman M M, Gray A I. Antimicrobial constituents from the stem bark of Feronia limonia.. Phytochemistry 2002; 59: 73–77
- Rahman M M, Gray A I. A benzoisofuranone derivative and carbazole alkaloids from Murraya koenigii. and their antimicrobial activity. Phytochemistry 2005; 66: 1601–1606
- Rahman M M, Sarker S D, Byres M, Gray A I. New salicylic acid and isoflavone derivatives from Flemingia paniculata.. J Nat Prod 2004; 67: 402–406
- Rao C P, Hanumaiah T, Vemuri V SS, Rao K VJ. Flavonol 3-glycosides from the leaves of Flemingia stricta.. Phytochemistry 1983; 22: 621–622
- Rao K N, Srimannarayana G. Fleminone, a flavanone from the stems of Flemingia macrophylla.. Phytochemistry 1983; 22: 2287–2290
- Rao K N, Srimannarayana G. Flemiphyllin, an isoflavone from stems of Flemingia macrophylla.. Phytochemistry 1984; 23: 927–929
- Saija A, Scalase M, Lanza M, Marzulla D, Bonina F, Castelli F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic Biol Med 1995; 19: 481–486
- Sarker S D, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro. antibacterial screening of phytochemicals. Methods 2007; 42: 321–324
- Subrahmanyam K, Rao J M, Vemuri V SS, Babu S S, Roy C P, Rao K VJ, Merlini L. New chalcones from leaves of Flemingia stricta Roxb (Leguminosae). Indian J Chem B 1982; 21: 895–897
- Yusuf M, Chowdhury J U, Wahab M A, Begum J. Medicinal Plants of Bangladesh. Bangladesh, BCSIR Laboratories, Chittagong 1994; 115