Abstract
Natural remedies from medicinal plants are considered to be effective and safe alternative treatments for diabetes mellitus. The current study deals with the effect of a single oral dose of the aqueous extract of Trichosanthes dioica. Roxb. (Cucurbitaceae) seeds in different diabetic animal models. Evaluation of the antihyperglycemic effect in normal, subdiabetic, and mild diabetic animal models is based on fasting blood glucose (FBG) and glucose tolerance test (GTT) studies. The graded doses of the extract, viz., 500, 750, 1000, and 1250 mg/kg body weight (b.w.), were administered orally. It was found that the blood glucose concentration decreased in a dose-dependent manner. The dose of 1000 mg/kg b.w. was found to be most effective with a maximum fall of 30.4% at 6 h during FBG studies in normal rats. However, the GTT studies showed the maximum reduction of 26.6% at 5 h in normal rats. Moreover, in case of subdiabetic and mild diabetic rats, the observed reduction in blood glucose levels was 32.8% and 35.9%, respectively, at 3 h during GTT. The data clearly reveal the significant antihyperglycemic profile of Trichosanthes dioica. seeds.
Introduction
Diabetes has been known since the time of the Ebers Papyrus and still remains a global burden. Diabetes mellitus is a syndrome due to different diseases characterized by a raised glucose concentration in the blood resulting because of failure of insulin secretion, impaired insulin action, or both (Atkinson & Maclaren, Citation1994; Teixeira et al., Citation2000; Yki-Jarvinen, Citation1994). The disorder is chronic and also affects the metabolism of fat and protein. There are two major types of diabetes. Type I, or insulin-dependent diabetes mellitus (IDDM) or juvenile onset, is conventionally treated with exogenous insulin. Type II, or non–insulin-dependent diabetes mellitus (NIDDM) or adult onset, is treated with oral hypoglycemic agents such as sulfonyl urea, biguanides, and so forth (Holman & Turner, Citation1991; Rosak, Citation2002). Complementary and alternative medicines are being widely used to treat diabetes mellitus (Payne, Citation2001). Herbal medicines have been found to be effective with nominal or no side effects (Gupta et al., Citation2005a; Kesari et al., Citation2005; Valiathan, Citation1998). In the natural system of medicine, many plants have been claimed to be useful for the treatment of diabetes mellitus (Alv et al., Citation1993; Grover et al., Citation2002; Gupta et al., Citation2005b; Irrova et al., Citation1989; Rai et al., Citation2007; Rao et al., Citation1997).
Trichosanthes dioica. Roxb. (Cucurbitaceae) is a dioecious perennial herbaceous vegetable. It is commonly known as “sespadula” in English or “parwal” in Hindi. It is widely grown throughout India (Chakravarthy, Citation1982) and, to a lesser extent, in other parts of South Asia. Fruits of Trichosanthes dioica. are a rich source of vitamin C and minerals such as 9.0 mg Mg, 2.6 mg Na, 83.0 mg K, 1.1 mg Cu, and 17.0 mg S per 100 g. In addition, fruits and other parts such as leaves and tender shoots have been used in the indigenous system of medicine since ancient times (Sharma et al., Citation1989). Recently, in normal animals, some specific medicinal properties have also been identified, viz., hypocholesterolemic, hypoglyceridimic, and hypophospholipidemic, by mixing shade-dried fruits of Trichosanthes dioica. in animal food (Mukharjee, Citation1996; Sharma & Pant, Citation1988a) and, also, by direct intake of fruit and pulp in normal and diabetic human volunteers (Sharma et al., Citation1989). Direct feeding of seeds of the plant was also found to be effective on serum lipid profile of normal and mild diabetic human subjects (Sharma et al., Citation1990) and albino rabbits (Sharma & Pant, Citation1988b). Effect of the seed fruit powder of Trichosanthes dioica. has also been studied on blood sugar and lipid profile of normal albino rabbits (Sharma & Pant, Citation1988c). Seeds of the plant were also found to possess antifungal and antibacterial activity and are widely used in the treatment of acid dyspeptic diseases (Harit & Rathee, Citation1996). Thus, the current investigation was undertaken to evaluate the glycemic profile of the aqueous extract of Trichosanthes dioica. seeds on blood glucose levels (BGLs) of normal and streptozotocin-induced subdiabetic and mild diabetic rats during fasting blood glucose (FBG) and glucose tolerance test (GTT) studies.
Materials and Methods
Preparation of crude drug
Fresh ripe fruits of Trichosanthes dioica. were purchased from the local market of Allahabad (India) and authenticated by Prof. Satya Narayan, Taxonomist, Department of Botany, University of Allahabad, India. A voucher specimen (AA/512/06) has been submitted. The fruits were cut into pieces, and the seeds were collected and shade-dried. The dried seeds (2 kg) were mechanically crushed and extracted with distilled water using a Soxhlet apparatus up to 48 h. The extract was filtered and concentrated in a rotatory evaporator at 35 ± 5°C under reduced pressure to obtain semisolid material, which was then lyophilized to get a powder (yield about 10.5% w/w).
Experimental animals
Male albino Wistar rats of the same age group and body weight (150–200 g) were selected for all the experiments. Animals obtained from the National Institute of Communicable Disease (NICD), New Delhi, India, were housed in polypropylene cages at an ambient temperature of 25–30°C and 45–55% relative humidity with a 12-h dark and light cycle. Animals were fed pellet diet (Golden Feed, New Delhi, India) and water ad libitum.. The study was approved by the institutional ethical committee (83 a/a/04/CPCSEA).
Induction of diabetes
Diabetes was induced by a single intraperitoneal injection of freshly prepared streptozotocin (50 mg/kg b.w.) in 0.1 M citrate buffer (pH = 4.5) to rats fasted overnight. After 3 days of streptozotocin (STZ) administration, rats with marked hyperglycemia (fasting blood glucose > 150 mg/dL) were selected for the study. The rats with hyperglycemia were divided into two groups: (1) subdiabetic animals with normal FBG and abnormal Postprandial glucose (PPG) levels, (2) mild diabetic animals with FBG 120–250/mg/dL.
Estimation
Blood glucose level was estimated by glucose oxidase method (Braham & Trinder, 1972) using a standard kit of Bayer Diagnostics India Limited, Baroda.
Experimental design
Initial screening of the aqueous extract for hypoglycemic activity was done with a range of variable doses in normal healthy rats by conducting FBG and GTT studies. The antidiabetic effect was assessed in subdiabetic as well as mild diabetic models with the same range of doses by studying their effect on FBG and GTT levels.
Assessment of hypoglycemic activity in normal healthy rats
Five groups of six rats each were used in the experiment. Group I served as untreated control and received vehicle (distilled water) only, and animals of groups II, III, IV, and V received aqueous seeds extract suspended in distilled water at doses of 500, 750, 1000, and 1250 mg/kg, respectively. Blood samples were collected from the tail vein at 2, 4, and 6 h after giving the extract.
Assessment of hypoglycemic activity by GTT in normal healthy rats
The aqueous extract was given orally to different groups of normal healthy animals in the same fashion as above, and its effect on FBG was studied hourly up to 2 h. The BGL value at 2 h was treated as “0” h value for GTT. The animals were then orally treated with 4 g/kg of glucose, and their glucose tolerance was studied at 1-h intervals for another 3 h. Thus, the total period of blood collection was up to 5 h.
Assessment of antidiabetic activity by GTT in subdiabetic and mild diabetic rats
The antidiabetic effect of the aqueous extract of Trichosanthes dioica. seeds in subdiabetic and mild diabetic rats was also assessed by improvement of glucose tolerance. The rats were divided in to six groups. Group I was the control, received vehicle (distilled water) only, whereas variable doses of 500, 750, 1000, and 1250 mg/kg of fruit extract was given orally to groups II, III, IV, and V, respectively. Blood glucose levels were checked first after 90 min of treatment, considered as “0” h value, and then 2 g/kg glucose was given orally to all the groups. Blood glucose levels were further checked up to 3 h at regular intervals of 1-h each, considered as 1-, 2-, and 3-h values. The results were compared with group VI of rats, which were treated with 250 mg/kg of tolbutamide (hypoglycemic agent).
LD50. experiment
Toxic effect of the water extract was also studied by a LD50 experiment. Two groups of rats of both sexes (six animals per group, three females and three males), weighing about 180–200 g, were orally treated by a single dose of 10 and 15 g of the aqueous extract of Trichosanthes dioica. seeds. Then, rats were observed for gross behavioral neurologic, autonomic, and toxic effects continuously. Food consumption, feces, and urine were also examined at 2 h and then at 6-h intervals for 24 h.
Statistical analysis
The data, expressed as the mean ± SD from six rats in each groups, were statistically evaluated using Student's t.-test followed by ANOVA.
Results
Effect on normal healthy rats
describes the hypoglycemic effect of a single oral of 500, 750, 1000, and 1250 mg/kg of aqueous seed extract in normal healthy rats. Treated rats showed a regular fall of 19.7%, 23.2%, and 30.4% from the doses of 500, 750, and 1000 mg/kg, respectively, after 6 h. However, a fall of only 27.1% was observed with the dose of 1250 mg/kg after the same interval of time.
Table 1 Effect of graded doses of Trichosanthes dioica. seed aqueous extract on BGLs of normoglycemic rats (mean ± SD).
Effect on normal healthy rats during GTT
deals with the study of aqueous extract of Trichosanthes dioica. seeds on BGL levels and glucose tolerance of normal healthy rats. Doses of 500, 750, 1000, and 1250 mg/kg of extract were given orally to overnight fasted healthy rats. The fall observed with doses of 500, 750, 1000, and 1250 mg/kg in BGL after 2 h of administration was 5.7%, 7.5%, 9.2%, and 8.2%, respectively, considered as “0” h value. However, the fall was observed up to 3 h after glucose administration at 1-h intervals, and the results reveal that the percentage fall in BGLs was regular up to the dose of 1000 mg/kg and reaches its maximum of 26.6%. Moreover, the fall of 25.4% was observed with the dose of 1250 mg/kg at the same time.
Table 2 Effect of graded doses of Trichosanthes dioica. seed aqueous extract on BGLs during GTT of normoglycemic rats (mean ± SD).
Effect on diabetic rats during GTT
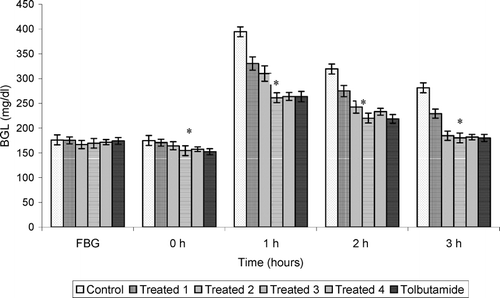
Figures 1 and 2 demonstrate the antidiabetic effect of aqueous extract of Trichosanthes dioica. seeds on subdiabetic and mild diabetic animals, respectively. Different doses of aqueous extract as mentioned above along with the standard drug tolbutamide (250 mg/kg) were given orally to the groups as defined in the experimental design. The fall of 22.5%, 25.1%, 32.8%, and 31.7% in BGLs of subdiabetic animals was observed after 3 h of glucose administration with doses of 500, 750, 1000, and 1250 mg/kg, respectively. However, the dose of 250 mg/kg of tolbutamide reduced BGL by 30.9% at 3 h during GTT in subdiabetic rats. Thus, the results clearly reveal that the dose of 1000 mg/kg of aqueous extract is more effective than the dose of 250 mg of tolbutamide during GTT in subdiabetic rats. The fall observed after 3 h of glucose administration was 18.5%, 34.4%, 35.9%, and 35.3% in BGLs of mild diabetic animals with the doses of 500, 750, 1000, and 1250 mg/kg, respectively. However, the dose of 250 mg/kg of tolbutamide reduced BGL by 36.0% at 3 h during GTT in mild diabetic rats, which is almost the same as with the dose of 1000 mg/kg in mild diabetic rats.
LD50 experiment
The experiment was carried out on normal healthy rats. The behavior of the treated rats appeared normal. No toxic effect was reported at doses up to 10-times and 15-times the effective dose of the aqueous extract, and there was no death in any of these groups.
Discussion
The current study was undertaken to assess the antihyperglycemic profile of aqueous extract of Trichosanthes dioica. seeds in albino Wister rats, and the study reveals a significant antihyperglycemic effect. The hypoglycemic and antilipidemic effect of feeding Trichosanthes dioica. whole fruits has been observed in normal albino rabbits (Sharma & Pant, Citation1988a) as well as in normal and diabetic human volunteers (Sharma et al., Citation1989). Direct feeding of seeds was also found to be effective on serum lipid profile of normal and mild diabetic human subjects (Sharma et al., Citation1990) and of albino rabbits (Sharma & Pant Citation1988b). Earlier, the study for the defined role of aqueous extract of Trichosanthes dioica. fruits on BGL in normal and diabetic rats, keeping in mind that the aqueous extract enhances the bioactivity, has already been carried out by our research group resulting in a positive response. Because, no data have been reported for evaluating the glycemic profile of aqueous extract of Trichosanthes dioica. seeds in STZ-induced animals, the current study was carried out as it has been observed a number of times that the seeds have much more activity than the fruits (Kamalakkannan et al., Citation2003, Citation2004; Kesari et al., Citation2006). The FBG and GTT studies were carried out on different types of models, viz., normal, subdiabetic, and mild diabetic. The maximum hypoglycemic effect (30.4%) was produced within 6 h during FBG studies in normal rats (). The GTT studies of normal rats clearly showed that the hypoglycemic effect was produced within 1 h, which increases further in the next hour considered as “0” h value (). This suggests that the active ingredients of the aqueous extract or their metabolites took about 1 h to exhibit their antihyperglycemic effect by reaching the target tissues through circulation. The data also shows that the effect remains significant even after 3 h of glucose administration.
The mechanism by which STZ brings about the diabetic state, close to type II diabetes of human, due to its cytotoxicity is the selective destruction of pancreatic, insulin-secreting β -cells of rats (Goldner & Gomori, Citation1943; Hofteizer, Citation1973). The aqueous extract of Trichosanthes dioica. seeds reduced the FBG levels significantly in STZ-induced diabetic rats. The GTT study of subdiabetic and mild diabetic animals showed marked improvement in glucose tolerance indicated by reduction in peak blood glucose levels. The dose of 1000 mg/kg of aqueous extract was found to be more effective than the dose of 250 mg/kg of the synthetic drug tolbutamide in case of subdiabetic animals. The same dose of aqueous extract shows almost similar effect as the dose of 250 mg/kg tolbutamide in case of mild diabetic animals. The effectiveness of the aqueous extract in both cases is comparable with the effectiveness of the synthetic drug tolbutamide.
Figure 1 Effect of graded doses of Trichosanthes dioica. seed aqueous extract on BGL during GTT in subdiabetic rats. *p < 0.001 compared with control. Control: distilled water. Treated 1, 500 mg/kg; Treated 2, 750 mg/kg; Treated 3, 1000 mg/kg; Treated 4, 1250 mg/kg; tolbutamide, 250 mg/kg.
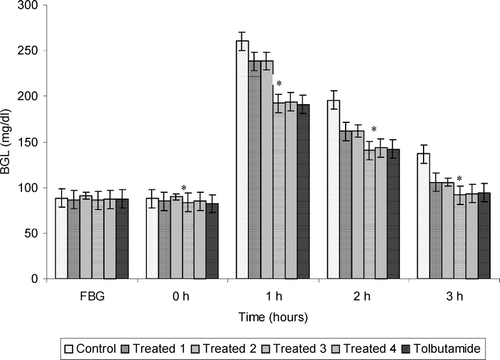
Figure 2 Effect of graded doses of Trichosanthes dioica. seed aqueous extract on BGL during GTT in mild diabetic rats. *p < 0.001 compared with control. Control, distilled water. Treated 1, 500 mg/kg; treated 2, 750 mg/kg; Treated 3, 1000 mg/kg; Treated 4, 1250 mg/kg; tolbutamide, 250 mg/kg.
In conclusion, the Trichosanthes dioica. seed extract demonstrates a high antihyperglycemic profile. The study has its own significance and relevance for diabetic persons. Its high LD50 indicates a great margin of safety for human subjects. Further characterization of active components in Trichosanthes dioica. seeds is warranted, and studies are in progress to isolate, identify, and characterize the active components.
Acknowledgment
The authors are thankful to Indian Council of Medical Research (ICMR) for financial assistance and to Mr. Devendra K. Rai, Department of Biochemistry, University of Allahabad, for providing ANOVA facility for statistical calculations. The first author (PKR) thanks ICMR for the award of SRF.
References
- Alv L, Azad Khan A K, Mamun M IR, Mosihuzzaman M, Nahar N, Nur-E-Alam M, Rokeya B. Studies on the hypoglycemic effects of fruit pulp, seeds, and whole plant of Momordica charantia. on normal and diabetic model rats. Planta Med 1993; 59: 408–412
- Atkinson M A, Maclaren N K. The pathogenesis of insulin dependent diabetes mellitus (review). New Engl J Med 1994; 331: 1428–1436
- Brahm D, Trinder P. Estimation of glucose-by-glucose oxidase method. Analyst 1972; 97: 142–145
- Chakravarthy H M. Fascicles of Flora of India-11 Cucurbitacae. Botanical Survey of India, New Delhil 1982; 136
- Goldner M, Gomori N. Alloxan induced diabetes. Endocrinology 1943; 33: 297–299
- Grover J K, Yadav S, Vats V. Medicinal plants of India with antidiabetic potential. J Ethnopharmacol 2002; 81: 81–100
- Gupta R K, Kesari A N, Murthy P S, Chandra R, Tandan V, Watal G. Hypoglycemic and anti-diabetic effect of ethanolic extract of leaves of Annona squamosa. L. in experimental animals. J Ethnopharmacol 2005a; 99: 75–81
- Gupta R K, Kesari A N, Watal G, Murthy P S, Chandra R, Tandan V. Hypoglycemic and anti-diabetic effect of aqueous extract of leaves of Annona squamosa. (L). in experimental animals. Curr Sci 2005b; 88: 1244–1254
- Harit M, Rathee P S. Antifungal activity of unsaponifiable fraction of the fixed oil of (Trichosanthes.) seed. Asian J Chem 1996; 8: 180–182
- Hofteizer V. Comparison of streptozotocin-induced diabetes in the rat inducing volumetric quantitation of the pancreatic islets. Diabetologia 1973; 9: 178–184
- Holmann R R, Turner R C. Oral Agents and insulin in treatment of NIDDM. Textbook of Diabetes, J Pickup, G Wiliams. Blackwell, Oxford 1991; 407–469
- Irrova M D, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol 1989; 27: 243–275
- Kamalakkannan N, Prince P SM. Hypoglycemic effect of water extract of Aegle marmelos. fruits in streptozotocin diabetic rats. J Ethnopharmacol 2003; 87: 207–210
- Kamalakkannan N, Prince P SM. Antidiabetic and antioxidant activity of Aegle marmelos. extract in streptozotocin induced diabetic rats. Pharm Biol 2004; 42: 125–130
- Kesari A N, Gupta R K, Singh S K, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos. seed extract in normal and diabetic rats. J Ethnopharmacol 2006; 107: 374–379
- Kesari A N, Gupta R K, Watal G. Hypoglycemic effects of Murraya koenigii. on normal and alloxan diabetic rabbits. J Ethnopharmacol 2005; 97: 247–251
- Mukharjee S K. Scope and use of indigenous herbal drugs in ‘Madhumeha’ An Indian scenario. International Seminar on Free Radicals, Mediated Diseases, VaransiIndia, September, 2–4, 1996, (Abstract 11)
- Payne C. Complementary and integrative medicine: Emerging therapies for diabetes part I. Diabetes Spec 2001; 14: 129–131
- Rai P K, Rai N K, Rai A K, Watal G. Role of LIBS in elemental analysis of P. guajava. responsible for glycemic potential. Inst Sc Tech 2007; 35(5)507–522
- Rao K, Giri R, Kesavulu M M, Apparao C. Herbal medicine in management of diabetes mellitus. Manphar Vidya Patrica 1997; 1: 33–35
- Rosak C. The pathophiologic basis of efficacy and clinical experience with the new oral antidiabetic agents. J Diab Comp 2002; 16: 123–132
- Sharma G, Pant M C. Effect of feeding Trichosanthes dioica. (Parval) whole fruits on blood glucose, serum triglycerides, phospholipid, cholesterol, and high density lipoprotein-cholesterol levels in the normal albino rabbits. Curr Sci 1988a; 57: 1085–1087
- Sharma G, Pant M C. Preliminary observations on serum biochemical parameters of albino rabbits fed on seeds of Trichosanthes dioica. (Roxb). Ind J Med Res 1988b; 87: 398–400
- Sharma G, Pant M C. Effect of raw deseeded fruit powder of Trichosanthes dioica.(Roxb) on blood sugar, serum cholesterol, high density lipo-protein, phaspholipid and triglycerides levels in the normal albino rabbits. Ind J Physiol Pharmacol 1988c; 32: 161–163
- Sharma G, Pandey D N, Pant M C. Biochemical evaluation of feeding Trichosanthes dioica. seeds in normal and mild- diabetic human subjects in relation to lipid profile. Ind J Physiol Pharmacol 1990; 34: 146–148
- Sharma G, Sarkar A, Pachori S B, Pant M C. Biochemical evaluation of raw Trichosanthes dioica. whole fruit and pulp in normal and mild- diabetic human volunteers in relation to lipid profile. Ind Drug 1989; 27: 24–28
- Teixeira C C, Rava C A, Dasilva P M, Melchior R, Argenta R, Anselmi F, Almedia C RC, Funchs F D. Absence of antihyperglycemic effect of Jambolon. in experimental and clinical models. J Ethnopharmacol 2000; 71: 343–347
- Valiathan M S. Healing plants. Curr Sci 1998; 75: 1122–1126
- Yki-Jarvinen H. Pathogenesis of non-insulin-dependent diabetes mellitus (Review). Lancet 1994; 343: 91–95
