Abstract
Given the high probability of developing cancer over the period of a normal life span, cancer chemoprevention provides an attractive therapeutic strategy for the delay or reversal of this process. A variety of phytochemicals, such as sulfides, isothiocyanates, glucosinolates, flavonoids, carotenoids, phenols, and diarylhepanoids, are known to mediate chemopreventive responses. Resveratrol, a ubiquitous stilbene found in the diet of human beings (e.g., as a component of grapes and wine), was uncovered by bioassay-guided fractionation and found to mediate cancer chemopreventive activity in a murine model with mechanisms involving various stages of the carcinogenic process. This work spurred a myriad of studies that are summarized in this article. As demonstrated with in vitro. and cell culture models, resveratrol functions through a plethora of mechanisms, which can vary from model to model. Results from differential gene expression studies are daunting. Irrespective of the precise mechanism, however, efficacy has been demonstrated in some animal models, and a critical evaluation of resveratrol data relative to the characteristics of a promising cancer chemopreventive agent leads to favorable consideration. Animal studies have shown cancer inhibitory activity in a number of models, including adenoma, skin, breast, colon, esophagus, glioma, intestinal, liver, and neuroblastoma. Biomarkers are known, and ample quantities of compound can be produced. Dietary administration is feasible. Several small-scale human trials are under way, and human intervention trials may follow. As learned by past experience, data from these trials are necessary prior to drawing any conclusions, but the current cancer chemopreventive profile of resveratrol provides promise for widespread use in the future.
Introduction
A summarized by the World Health Organization, cancer leads to about 12% of human deaths (Citation2), claiming more than 10,000,000 lives each year. In the United States, cancer is the second leading cause of death, being responsible for approximately one in every four deaths. Interestingly, it is believed that at least one-third of all cancers could be prevented (Citation3, Citation4). As such, primary and secondary prevention strategies are reasonable approaches to reduce the occurrence of this disease (Citation5, Citation6, Citation7) and subsequent deaths. Primary prevention strategies involve removing causative agents and other life style modifications that decrease the risk of cancer, as exemplified by smoking cessation and screening tests to detect precancerous lesions. Unfortunately, not all causative agents are known, and other suspected carcinogens are too widespread to prevent all exposure.
Secondary prevention, cancer chemoprevention, involves the use of nontoxic natural and/or synthetic agents to decrease the risk of malignant tumor development or spread (Citation8, Citation9). Cancer chemoprevention is a multidisciplinary field of research that has evolved from numerous scientific observations (Citation10). For example, epidemiologic studies have linked diets high in fresh fruits and vegetables to lower cancer rates. This dietary link is perhaps most strongly supported by studies reporting the cancer risk of migrants from areas of low incidence to high incidence. These studies demonstrated that the incidence of cancer among children of migrants is similar to that of the general population (Citation11).
Another important breakthrough has been the prevention of experimentally induced cancer in laboratory animals. It was subsequently postulated that dietary components, particularly specific nutrients and/or phytochemicals found in fruits and vegetables, could be used to prevent cancer in human beings (Citation9, Citation12). More recently, research in cancer biology has elucidated molecular mechanisms of cancer chemopreventive agents (Citation6, Citation10, Citation13). Much of the theoretical basis for cancer chemoprevention is the understanding that cancer develops over time through the process of carcinogenesis (Citation14). This process has been broken down into distinct yet overlapping stages, namely, initiation, promotion, and progression. The evolution of these stages is believed to take 10 to 40 years, during which various genetic mutations must occur (Citation10, Citation15). The field of cancer chemoprevention is focused on reversing, halting, or delaying these stages of carcinogenesis by means of secondary prevention (Citation8, Citation9, Citation10).
Cancer chemopreventive agents have been classified according to the stage of carcinogenesis in which they have demonstrated activity and are broadly termed blocking. and suppressing. agents (Citation8). Blocking agents act by preventing the initiation stage through a variety of mechanisms such as directly detoxifying carcinogens, stimulating detoxifying enzymes, and inhibiting carcinogen formation. Suppressing agents act at the promotion and progression stages through mechanisms such as inhibition of arachidonic acid metabolism, induction of cell differentiation, and inhibition of ornithine decarboxylase activity (Citation4, Citation8, Citation16). In the case of hormone-dependent cancers, suppressing agents may act by preventing the hormone from binding to its receptor, as exemplified by the use of the selective estrogen receptor modulators, tamoxifen and raloxifene, for breast cancer prevention (Citation4, Citation15).
Overview of Cancer Chemoprevention Trials Involving Phytochemicals
Many early cancer chemoprevention studies were focused on nutrients such as vitamin C, calcium, and retinoids (Citation9, Citation11). In the past several decades, nonnutrient phytochemicals found in fruits and vegetables have been examined, and a number of promising natural product leads have resulted from this research effort (Citation15, Citation17, Citation18). For example, green tea extract and pure compounds such as caffeic acid phenethyl ester, capsaicin, curcumin, 6-gingerol, indole-3-carbinol, lycopene, and perillyl alcohol are undergoing clinical trials for their cancer chemopreventive activities (Citation15, Citation19, Citation20). The U.S. National Cancer Institute is supporting the evaluation of potential cancer chemopreventive agents at different levels of preclinical development and clinical trials (Citation18). Examples of natural products currently under preclinical or clinical development for cancer chemoprevention include curcumin and lycopene, which are in a phase I study for the prevention of colon cancer, and a soy protein supplement is in a phase II trial for the prevention of prostate cancer in patients with elevated prostate-specific antigens (Citation21). Moreover, soy isoflavones are also involved in a randomized study in preventing further development of cancer in patients with stage I or stage II prostate cancer (Citation21). Polyphenon E (green tea extract), in combination with low-dose aspirin, is in a phase II randomized study to prevent cancer in women at high risk for developing breast cancer (Citation22, Citation23). Other natural products currently being investigated include S.-allyl-l-cysteine, epigallocatechin gallate, genistein, folic acid, and quercetin (Citation19, Citation24).
Discovery and Characterization of Natural Product Inhibitors of Carcinogenesis
With support provided by the National Cancer Institute, we have conducted a program project entitled “Natural Inhibitors of Carcinogenesis” since 1991. The major aim of this project has been the discovery of new cancer chemopreventive agents from plants, particularly those that are edible. We are now beginning to explore marine microorganisms for chemopreventive activity. The project involves botanical, biological, chemical, biostatistical, and administrative aspects (Citation25, Citation26, Citation27, Citation28). Terrestrial plant materials selected for investigation are prioritized based on information obtained from the NAPRALERT database (Citation29). Edible plants or species with reported biological activity related to cancer chemoprevention, plants with no history of toxicity, and those poorly investigated phytochemically are selected for preliminary investigation, and a small amount of plant material is collected (Citation25, Citation26, Citation27).
The panel of in vitro. bioassays used for the discovery of potential cancer chemopreventive drugs includes screening tests that are typically enzyme-or cell-based assays (Citation26, Citation30). These assays are adapted to high-throughput measurement techniques performed relatively rapidly in order to uncover the biological properties of a large number of candidate substances (Citation26, Citation30). The initial bioassays afford a strategic framework for the evaluation of agents according to defined criteria, to provide evidence of agent efficacy, and to serve to generate valuable dose-response, toxicity, and pharmacokinetic data required prior to phase I clinical safety testing (Citation26, Citation30, Citation31).
Thus, preliminary screening is performed with an ethyl acetate–soluble partition extract using a battery of short-term in vitro. bioassays (Citation26). Bioactive extracts are further evaluated in a mouse mammary organ culture (MMOC) model as a secondary discriminator (Citation32, Citation33). The battery of short-term in vitro. assays was developed to monitor tumorigenesis at different stages. For example, antimutagenicity activity, antioxidant activity, and induction of NADPH:quinone reductase activity has been monitored to evaluate inhibition of carcinogenesis at the initiation stage (Citation34, Citation35, Citation36, Citation37). Monitoring inhibition of carcinogenesis at the promotion stage has been performed by evaluating the inhibition of phorbol ester–induced ornithine decarboxylase activity, inhibition of cyclooxygenase-1 and-2 activity, inhibition of phorbol dibutyrate receptor binding, and inhibition of transformation of JB6 mouse epidermal cells (Citation38, Citation39, Citation40, Citation41). Induction of HL-60 human promyelocytic leukemia cell differentiation and inhibition of aromatase, antiestrogenic, estrogenic, and estrone sulfatase activities have been used to monitor inhibition of carcinogenesis at the progression stage (Citation42, Citation43, Citation44, Citation45). Various additional assays are under development, such as inhibition of quinone reductase 2, RxR, NF-κ B, and Keap.
Plant extracts showing potency and/or selectivity in preliminary biological screening procedures are selected for bioassay-guided fractionation to isolate the active principle or principles. Crude methanol extracts are partitioned using solvents of varying polarities and then chromatographed by either gravity-, flash-, or low-pressure column over silica, alumina, ion-exchange resins, polyamide, reversed-phase silica gel, size-exclusion gels, or other solid-phase supporting material (Citation27, Citation46). Analytical thin-layer and high-pressure liquid chromatography (HPLC) techniques are used to help determine optimal solvent systems for the maximal separation of active components of fractions (Citation47). Other separation techniques, such as droplet countercurrent chromatography (DCCC), high-speed countercurrent chromatography (HSCCC), and semipreparative HPLC are used occasionally for complex mixtures of active constituents (Citation27, Citation47, Citation48). A more innovative procedure using LC-MS/MS has been devised (Citation49).
After pure active isolates are evaluated in all of the available in vitro. assays, selected compounds are evaluated in the ex vivo. mouse mammary organ culture model (Citation32, Citation33). Highly promising leads may be selected for testing in full-term animal tumorigenesis models, such as the two-stage mouse skin model using 7,12-dimethylbenz(a.)anthracene (DMBA) as initiator and 12-O.-tetradecanoylphorbol 13-acetate (TPA) as promoter, and in the rat and mouse mammary carcinogenesis models with DMBA or N.-methyl-N.-nitrosourea (MNU) as the carcinogens (Citation25, Citation26, Citation40). Other animal models may be used as well.
Potential Cancer Chemopreventive Agents from Plants
As an example of the success of this program, over a recent period of approximately 5 years, a total of 166 active compounds were isolated and biologically evaluated in our laboratories from 32 plant species (Citation50). The active metabolites were obtained using activity-guided fractionation with a preselected in vitro. assay to monitor their purification process. These active compounds were found to represent 29 major secondary metabolite compound classes including alkaloids (of the β-carboline alkaloid, indoloquinoline alkaloids, and steroidal types), amides, benzenoids, benzofurans, cardiac glycosides, ceramides, a coumarin, diarylheptanoids, diterpenoids, fatty acids, flavonoids (of the aurone, bisaurone, chalcone, flavan, flavanone, flavone, flavonol, flavonone, and isoflavone types), glycerin esters, a β-ionone derivative, an iridoid, lignans, a monoterpenoid, a naphthopyran, norwithanolides, phenylphenalones, a porphyrin derivative, a rocaglamide derivative, rotenoids, sesquiterpene lactones, sesquiterpenoids, simaroubolides, a stilbenolignan, stilbenoids, triterpenoids, and withanolides. Active compounds based on three different types of novel carbon skeletons were obtained during this work, which included seven norwithanolides possessing a new C27 skeleton (as opposed to the 28 carbons of the more widespread withanolides) (Citation47, Citation51), a novel stilbenolignan containing a stilbene-phenylpropane unit with a dioxane moiety (Citation52), and two triterpenes based on a 29-nor.-3,4-seco.-cycloartane skeleton (Citation53). Forty-nine new compounds from 19 species were found among the compound classes mentioned above and were classified into 16 major structural classes. A large number of known bioactive compounds were isolated from 32 species and can be grouped into 23 major structural classes. As summarized in , nine agents resulting from this project are considered promising leads for further development.
Table 1 Selective chemopreventive agents identified from natural products.Footnotea.
The Phenomenon of Resveratrol
One of the most fascinating molecules we have “rediscovered” is resveratrol (). Resveratrol is a natural phytoalexin that is expressed in plants as a defensive response against fungal infections and other environmental stressors (Citation65). The word alexin. is from the Greek language, meaning “to ward off” or “to protect.” Resveratrol may also have alexin-like activity in human beings, protecting against degenerative diseases. Synthesis of resveratrol in grapes is most likely associated with natural stress factors such as exposure to ultraviolet radiation (Citation66), injury, or during fungal or mold invasion (Citation67). Significant amounts of resveratrol were detected in healthy fruit clusters prior to any detectable mold lesions. This suggested that the compound was biosynthesized soon after the recognition of the pathogen by the plant (Citation68). Montero et al. (Citation69) investigated involvement of the plant hormone ethylene in resveratrol synthesis during fruit maturation. High resveratrol content correlated with low ethylene emission. Exogenous application of resveratrol on the fruit surface delayed the increase of ethylene emission and doubled the normal shelf-life of grapes. This response is due to the antifungal activity of resveratrol, indicating the wide potential of such a compound for the control of the microbiota on fruits and practical application as a natural chemical to prolong the shelf-life of fruits (Citation70).
Resveratrol was first recognized as a biologically active compound by Siemann and Creasy (Citation71). The compound is found in several plants, chiefly in red grapes. The highest concentration (50–100 μ g/g of grape wet weight) was determined in the grape skin. In wine, cis.-and trans.-isomers are present, in the free or glycosylated forms. cis.-Resveratrol was not detected in grape skin and juices. Formation of the cis.-isomer by isomerization or breakdown of the trans.-form on exposure of wine to light and oxygen has been assumed (Citation72). In dietary supplements, the isomer is not always specified, but in most cases it is the trans.-form. In red wine, the concentration of the trans.-isomer ranges between 0.1 and 15 mg/L. The ratio of cis.-and trans.-resveratrol in wines varies by region. Climate, the type of grape, and the length of time the skin is kept with the grape during the winemaking process are some factors that influence the level of resveratrol and the ratio of isomers in wine (Citation73). Primarily, the compound is produced in the grape, grape shoots, and vines. Increasing irradiation of harvested grapes by UVB or UVC light enhances yields of resveratrol (Citation72). Most resveratrol-containing supplements marketed in the United States. contain extracts of the root of Polygonium cuspidatum. Sieb. and Zucc., also known as the Japanese knotweed. The dried root and stem of this plant is used in traditional Japanese folk medicine (Ko-jo-kon) as a circulatory tonic, against fungal diseases, and for various inflammatory and liver diseases (Citation74). Moreover, resveratrol synthase genes have been isolated and inserted into plants, creating transgenic varieties of alfalfa, tobacco, and other plant species with higher trans.-resveratrol concentrations. Phytoalexins inserted into plants may provide defense against different pathogens (Citation75). Additionally, transgenic plants (e.g., alfalfa) transformed with resveratrol synthesizing genes might become an economical source of the compound for scientific research or dietary supplements (Citation76).
As part of our search for natural product cancer chemopreventive agents, acquisition number 46 (the current total is 7148), a nonedible legume identified as Cassia quinquangulata. Rich. (Leguminosae), was extracted and found to demonstrate impressive inhibition with cyclooxygenase-1. Activity was also observed in the mouse mammary organ culture (MMOC) model, and the extract was selected for bioassay-guided fractionation. As a result, resveratrol was readily identified as the active principle. In addition to inhibiting cyclooxygenase activity, suggestive of antipromotional activity, the isolate was found to serve as an antioxidant and antimutagen. Further, it induced phase II drug metabolizing enzymes involved chiefly in the detoxification of carginogen metabolites (anti-initiation activity) and induced human promyelocytic leukemia cell differentiation (antiprogression activity). Finally, antitumor and anti-inflammatory effects were observed with mouse and rat models, respectively, providing support for the physiologic significance of the in vitro. and cell culture data (Citation40).
When these data were published in 1997, a search of MEDLINE revealed a total of 21 articles in the literature, largely relating to the natural occurrence of resveratrol rather than to biologic potential. There was a huge response by the media and public, perhaps because it was otherwise a slow news day, but clearly the public found comfort in the notion of food and beverages (such as grapes and wine) being of benefit for their health. Obviously, in addition to the general population, this notion attracted the attention of the scientific community. As indicated by a recent query of MEDLINE, from 1997 to the present, a total of 1974 articles investigating resveratrol have been published (). Symposia have been conducted (Citation77), funding streams have been created (California Table Grape Commission, http://www.freshCaliforniagrape.com/), companies have been formed (Royalmount Pharma, http://www. royalmountpharma.com/; Sirtris Pharmaceuticals Inc., http://www.sirtrispharma.com/), various commercial products are available (), patents have been granted (), and monographs and reviews have been written () (Citation78, Citation79, Citation80, Citation81, Citation82, Citation83, Citation84). Of some importance, because this molecule is not complex, facile chemical syntheses have been devised, so abundant supplies of resveratrol are available (Citation174, Citation175, Citation176, Citation177, Citation178).
Figure 2 Line chart mapping the number of publications dealing with resveratrol from 1975 to July 2007.
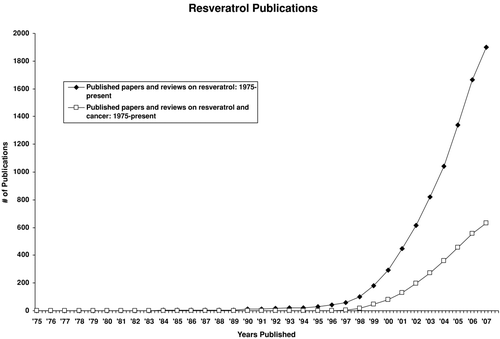
Table 2 Selection of commercial sources of resveratrol.
Table 3 Select U.S. patents for the use of resveratrol as related to cancer.
Table 4 Review articles.
In this review, a synopsis of the literature describing the cancer-related activity of resveratrol will be presented. The results are presented in tabular form, roughly divided into reports studying resveratrol with in vitro. models, cell culture systems, in vivo. systems, and clinical trials. In studies wherein multiple models were employed, the article is listed in the table representing the highest level of biological complexity.
In Vitro. Studies Conducted with Resveratrol
Relatively few reports have appeared wherein the primary tests were performed mainly with in vitro. model systems. Some are presented in . Key observations have demonstrated antioxidant activity (Citation35, Citation181) and ability to inhibit protein kinases (Citation186, Citation188, Citation189), cyclooxygenases (Citation179, Citation199, Citation200), cytochromes P450 (Citation190), and tyrosinase (Citation183). Cr-induced damage to DNA can be prevented, probably through radical scavenging (Citation184). Metabolism has also been established, through glucuronidation and sulfonation, conversion to piceatannol, and metabolism can be modulated by flavonoids such as quercetin (Citation182, Citation185, Citation187). Mammalian proteins capable of binding resveratrol have been identified (Citation120, Citation180). The crystal structure of resveratrol bound to the active site of cyclooxygenase as been deduced (Mesecar et al., submitted for publication), indicating some precise mechanisms are beginning to be defined.
Table 5 Evaluation of resveratrol with in vitro. model systems.
Cell Culture Studies Conducted with Resveratrol
Clearly, as summarized in , the majority of studies that have been performed to investigate the mode of resveratrol action involve cultured cells. In our original report (Citation40), HL-60 and Hepa 1c1c7 cells were used, and these responses have been confirmed and expanded. As models of human cancers, prostate, colon, lung, breast, ovarian, renal, hepatoma, leukemic, bronchial, neuroblastoma, cervical, lymphoma, medulloblastoma, endometrial, esophageal, melanoma, pancreatic, gastric, epidermal, thyroid, fibroblast, retinoblastoma, and squamous cells as well as macrophages, monocytes, myofibroblasts, transformed and transfected cells, and organ culture systems have been used. Activities may vary from system to system, but some generalizations apply. Certainly, apoptosis is a common mode of action, and the response is generally dependent on p53 (Citation223, Citation225, Citation242, Citation254, Citation280, Citation289, Citation298, Citation300, Citation326, Citation348). A number of related factors can be modulated by resveratrol, such as activation of caspases, decreases in Bcl-2 and Bcl-xL., increases in Bax, inhibition of S-type cyclins and cyclin-dependent kinases, activation of c-jun NH2-terminal kinase, and interference with NF-κ B and AP-1 mediated cascades. Of course, nonapoptotic cell death pathways have also been observed, as well as induction of cell differentiation (Citation40, Citation292). In some cases, p53-independent apoptosis has been reported (Citation241, Citation309).
Table 6 Evaluation of resveratrol with cell culture model systems.
A number of studies have demonstrated the potential of resveratrol to inhibit cell invasion (Citation320, Citation321, Citation366, Citation382, Citation387, Citation395, Citation412, Citation418, Citation421, Citation435, Citation440) and angiogenesis factors (Citation215, Citation430, Citation444). Cell transformation can be blocked (Citation264, Citation348, Citation351). As with in vitro. studies, ribonucleotide reductase (Citation281, Citation350), cyclooxygenases (Citation79, Citation449), i.NOS (Citation80, Citation220, Citation293), and various kinases (Citation240, Citation244, Citation248, Citation266, Citation310) are inhibited. The compound can function as an antioxidant (Citation354, Citation428) or a prooxidant (Citation377, Citation402, Citation420, Citation436, Citation438). Cathepsin D is regulated (Citation379), hypoxia-induced protein is inhibited (Citation387), and telomerase is downregulated (Citation375). Various enzymes are modulated (cf. Citation308, Citation365, Citation431), as is polyamine metabolism (Citation116, Citation265). Clearly, a large number of mechanisms have been explored.
In hormone-responsive cell types, a variety of studies have been performed to assess the hormonal (estrogenic or androgenic) potential of resveratrol (Citation213, Citation226, Citation231, Citation234, Citation237, Citation262, Citation269, Citation278, Citation285, Citation317, Citation331, Citation346, Citation352), largely due to the structural similarity with diethylstibestrol (DES). Data range from superagonistic in transient transfection studies with reporter genes to completely inactive. The compound has been described as an estrogen (Citation213, Citation231, Citation269, Citation278, Citation352) and an antiestrogen (Citation307, Citation317, Citation346, Citation412). No binding (Citation372) or low binding (Citation317, Citation331) has been observed with estrogen receptors. Activity can be mediated in ER cell lines (cf. Citation433); androgen receptor (Citation340) and PSA levels (Citation270, Citation337) can be reduced. This remains a somewhat controvercial topic. Most typically, however, weak hormonal activity has been observed in the absence of the native steroid, and antihormonal activity has been observed with the addition of native hormone.
As might be expected, further studies have been performed to investigate structural derivatives of resveratrol, either naturally occurring stilbenes or synthetic analogues, as well as cis.-and trans.-isomers (Citation178, Citation199, Citation200, Citation212, Citation245, Citation249, Citation255, Citation257, Citation261, Citation264, Citation285, Citation288, Citation294, Citation301, Citation304, Citation308, Citation311, Citation327, Citation363, Citation386, Citation407, Citation425, Citation426). These data are of interest, as is the generation and subsequent biologic potential of resveratrol metabolites and results obtained with cell culture models of transport (Citation124, Citation233, Citation265, Citation276). In the area of cancer chemotherapy, the ability of resveratrol to modulate the toxic side effects of dacarbazine, taxol, vincristine, vinorelbine, cyclosporin A, retinoic acid, 5-fluorouracil, and so forth, have been investigated (Citation236, Citation238, Citation250, Citation272, Citation303, Citation325, Citation367, Citation380, Citation388, Citation392, Citation419, Citation424, Citation450). In addition, several studies have illustrated enhanced radiation-induced cell death in the presence of resveratrol (Citation229, Citation306, Citation378, Citation429, Citation432). Further, effects in combination with various other agents such as quercetin have been examined (Citation284, Citation291, Citation339, Citation347, Citation422, Citation423).
The overall mechanism that is facilitated by resveratrol is undoubtedly complex. As demonstrated by differential expression studies in various cell cultures (Citation216, Citation268, Citation270, Citation295, Citation416), hundreds of genes are affected by treatment with resveratrol. These results are quite profound and are consistent with the raft of responses observed in numerous model systems. The overall physiologic significance remains to be defined (Citation451).
In Vivo. Studies Conducted with Resveratrol
On an intuitive level, data obtained with studies performed with in vivo. models appear to be of greatest relevance to the human situation: “the proof is in the pudding.” It was clear from the outset that resveratrol is capable of mediating physiologic responses in animal models. In our original report (Citation40), anti-inflammatory activity was observed in rats, and inhibition of tumorigenesis was observed in the two-stage mouse skin model. Importantly, in the rat inflammation model, resveratrol was administered orally, so a preliminary indication of bioavailability and systemic activity was also provided.
Clearly, however, experimental outcomes are dependent on the particular model and protocol that is applied. Inhibition in the two-stage mouse skin system has been confirmed (Citation452, Citation453) and greatly expanded with activity being observed in UV-induced skin cancer models (Citation454, Citation455, Citation456, Citation457). These data are very promising and suggest utility for the prevention of skin cancer.
As a logical extension of the numerous mechanistic studies performed with cell culture models described above, many animal studies have been reported in the literature (). In part, these studies have been designed to examine some biomarkers of carcinogenesis (Citation464, Citation483, Citation496, Citation501), a few derivatives of resveratrol (Citation465, Citation491), and to investigate absorption and metabolism (Citation465, Citation474, Citation478, Citation507). In addition, of course, a variety of antitumor models have been employed. With mice, resveratrol reduced biomarkers of lung carcinogenesis produced in benzo(a.)pyrene-treated mice (Citation471) but not tumorigenesis (Citation461). We also found that resveratrol was not active in the benzo(a.)pyrene mouse lung tumorigenesis model (unpublished data), nor was it active in a mixed-carcinogen lung cancer model (Citation487). A positive response was observed, however, with Lewis lung carcinoma–bearing mice (Citation485). This response may have been due to an anti-angiogeneric response mediated by resveratrol (Citation485), as has been noted in various other antitumor models (Citation493, Citation497, Citation500).
Table 7 Evaluation of resveratrol with in vivo. model systems.
In one study, a lack of activity was observed in the Min. mouse (Citation466), but similar studies reported a reduction of intestinal tumors when resveratrol was tested in this model (Citation484, Citation503). Aberrant crypts were also reduced in carcinogen-treated rats (Citation486), as were colon tumors in DMH-treated rats (Citation498).
An increase in tumorigenesis was reported when resveratrol was administered to rats treated with N.-methyl-N.-nitrosourea (Citation468), but this is contrary to our results wherein an inhibition was observed (Citation483). Activity was also reported in two studies conducted with the DMBA rat mammary carcinogenesis model (Citation476, Citation489), as well as the HER-2/new spontaneous breast cancer model (Citation502). A positive response was also reported with the NMBA esophageal model (Citation477), as well as with some combination regimens (Citation450, Citation492). Finally, although activity was not observed with 4T1 breast cancer (Citation482), B16 melanoma (Citation478), and leukemia (Citation481), positive responses were demonstrated with tumor transplant models for hepatoma (Citation378, Citation450, Citation469, Citation472, Citation473, Citation488), neuroblastoma (Citation463), sarcoma (Citation467), pancreatic (Citation480), mammary (Citation497), lung (Citation485), glioma (Citation493), laryngeal (Citation499), and gastric (Citation504) cancers.
Conclusions
As summarized above, a great deal of work has been performed over the past several years to characterize the cancer chemopreventive and therapeutic potential of resveratrol. The ultimate objective of this work is to answer one question: Is resveratrol of value to alleviate any type of cancer in human beings? Because human beings are already consuming resveratrol, either as a constituent of the diet or as a dietary supplement, data could already exist to suggest the potential of resveratrol to function in this capacity. Consumption of red wine, for example, implies the ingestion of resveratrol, and correlations can be examined between consumption and cancer incident. However, no clear answers can be derived from such epidemiologic considerations, so the possible efficacy of resveratrol remains an open question. As was learned by the failure of β-carotene to prevent lung cancer (Citation508, Citation509), human clinical trials are necessary to understand the true efficacy of experimental agents, irrespective of compelling laboratory data that may suggest effectiveness.
Spearheaded by Waun Ki Hong and Michael B. Sporn, a Chemopreventive Working Group recently provided a report describing the prevention of cancer in the current millennium (Citation4). Included in this report was a list of seven desirable/acceptable characterizations of cancer chemopreventive agents. In brief, these will be considered in the context of resveratrol.
Efficacy in preventing cancer.. Resveratrol has been shown to demonstrate efficacy in multiple animal models. Activity in human beings is unknown.
Knowledge about mechanism of inhibition.. As summarized in this article, a great deal of information is available concerning the mechanism of action of resveratrol. Although a straightforward sequence of critical events cannot be defined due to the overtly pleiotropic mode of action, some existing data are certainly valuable.
Information as to likely efficacy in the human.. The most compelling data are derived from animal studies in which resveratrol is administered by the oral route. This has been accomplished. Therefore, although absorption and metabolism (Citation124, Citation233, Citation265, Citation276) requires additional investigation and remains moot to some extent, the potential of efficacy in humans does appear likely. Some indication of toxicity has been suggested (Citation277, Citation283) and needs to be further defined, but most studies suggest favorable therapeutic indices.
Demonstration of efficacy in experimental animals.. In general, it is not possible to predict efficacy in only one animal model. Efficacy has been demonstrated in breast, skin, esophagus, and colon models, but further tests should be performed in additional models such as bladder, prostate, uterus, and kidney. Resveratrol does not appear active in some mouse lung cancer models.
Lack of toxicity and undesirable side effects.. Certainly, long-term feeding studies have been performed with resveratrol without untoward toxic side-effects (Citation483), but some suggestions of potential toxicity and/or hormonal activity have been put forth. Overall, it seems highly likely that a therapeutic regimen could be devised with an acceptable risk/benefit ratio. Nonetheless, thorough preclinical assessment of resveratrol in acceptable models of toxicity will be required prior to advocating long-term human investigation trials.
Compounds already approved by the FDA for human use or likely to be approved readily.. To obtain FDA approval for clinical trails, it is likely that comprehensive preclinical toxicity trials are necessary. However, these studies are straightforward, and a notable advantage is an ample supply of resveratrol through chemical synthesis. The work needs to be completed prior to drawing conclusions, but existing data suggest acceptable dose regimens could be devised, and it seems likely that FDA approval would follow.
Occurrence of the agent in foods or beverages.. The occurrence of resveratrol in foods or beverages is an obvious advantage in terms of development. It can already be stated with a high degree of confidence that consumption of limited quantities of resveratrol is not harmful to human beings, and great flexibility becomes available in terms of long-term dosing strategies.
Consistent with these suggestions, some limited data are available from studies conducted with human beings, and four small-scale phase trials are under way (). First and foremost, as predicted from animal and cell culture studies, resveratrol is readily absorbed after oral administration and rapidly metabolized (Citation507, Citation510, Citation512). The primary metabolites are sulfates and glucuronides. These metabolites require further investigation as they are probably responsible for the biological response mediated by resveratrol administration. In addition, resveratrol is present, albeit in low concentrations, so physiologic responses would need to be facilitated by the parent compound with great specificity and avidy. A combination effect is feasible, especially as so many potential targets have been identified.
Table 8 Clinical and human trials.
In sum, a great deal of time, money, and intellectual capital has been invested in the exploration of resveratrol. From a purely academic point of view, considering the structural simplicity of resveratrol, the extent of this effort is incredible. Implicitly, however, the shear magnitude of investigation supports the intrinsic value of this compound. Based on the criteria discussed above, and the overall favorable characteristics of resveratrol, it is reasonable to advocate further development as a cancer chemopreventive agent. Perhaps this review will have some value in facilitating the process.
Acknowledgments
The author is grateful to faculty colleagues associated with this research project, namely, Drs. C-j. Chang, B. Craig, M. Cushman, W. Fenical, H.H.S. Fong, A.D. Mesecar, R.C. Moon, and R.B. van Breemen, and to many postdoctoral associates, graduate students, and research assistants who worked in the laboratory in support of this research. Special thanks are extended to Elizabeth Ryan for help in organizing this manuscript and collating the descriptions given in the tables. The support of collaborators throughout the world who have participated in the selection, collection, and identification of plant materials used in the current work is also gratefully acknowledged. The current work is supported by program project grant P01 CA48112, funded by the National Cancer Institute, NIH, Bethesda, Maryland, USA.
Notes
1This review has been revised, expanded, and updated from a previous chapter (see. Ref. 1) on the same subject.
2Due to the unique nature of this review, the format is atypical. The references are presented by number, rather than by author name and year, and the tables are presented at the end of the text, as they are so extensive.
References
- Pezzuto J. Resveratrol as an inhibitor of carcinogenesis. Resveratrol in Health and Disease, B Aggarwal, S Shishodia. Taylor & Francis, New York 2006; 233–383
- Stewart B, Kleihues P. World Cancer Report. IACR Press, Lyon 2003; 9–19
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun M. Cancer statistics. CA Cancer J Clin 2003; 53: 5–26
- Alberts D, Conney A, Ernster V, Garber J, Greenwald P, Gudas L, Hong W, Kelloff G, Kramer R, Lerman C, Mangelsdorf D, Matter A, Minna J, Nelson W, Pezzuto J, Prendergast F, Rusch V, Sporn M, Wattenberg L, Weinstein B. Prevention of cancer in the next millennium. Report of the Chemoprevention Working Group to the American Association for Cancer Research. Cancer Res 1999; 59: 4743–4758
- Kelloff G J. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res 2000; 78: 199–334
- Sporn M. The war on cancer. Lancet 1996; 347: 1377–1381
- Greenwald P, Kelloff G, Burch-Whitman C, Kramer B. Chemoprevention. CA Cancer J Clin 1995; 45: 31–49
- Wattenberg L. Chemoprevention of cancer. Cancer Res 1985; 45: 1–8
- Sporn M, Dunlop N, Newton D, Smith J. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogs (retinoids). Fed Proc 1976; 35: 1332–1338
- Kelloff G, Hawk E, Karp J, Crowell J, Boone C, Steele V, Lubet R, Sigman C. Progress in clinical chemoprevention. Semin Oncol 1997; 24: 241–252
- Willett W, MacMahon B. Diet and cancer: an overview [second of two parts]. N Engl J Med 1984; 310: 697–703
- Harris C. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res 1991; 51(Suppl)5023–5044
- Flora S D. Mechanisms of inhibitors of mutagenesis and carcinogenesis. Mutation Res 1998; 402: 151–158
- Sporn M. Carcinogenesis and cancer: Different perspectives on the same disease. Cancer Res 1991; 51: 6215–6218
- Kelloff G, Crowell J, Steele V, Lubet R, Boone C, Malone W, Hawk E, Lieberman R, Lawrence J, Kopelovich L, Ali I, Viner J, Sigman C. Progress in cancer chemoprevention. Ann NY Acad Sci 1999; 889: 1–13
- Morse M, Stoner G. Cancer chemoprevention: Principles and prospects. Carcinogenesis 1993; 14: 1737–1746
- Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res 1999; 428: 305–327
- Reddy L, Bhoola K. Natural products for cancer prevention: A global perspective. Pharmacol Ther 2003; 99: 1–13
- Fujiki H, Suganuma M, Imai K, Nakachi K. Green tea: Cancer preventive beverage and/or drug. Cancer Lett 2002; 188: 9–13
- Surh Y-J. Cancer chemoprevention with dietary phytochemicals. Nature Rev Cancer 2003; 3: 768–780
- ClinicalTrials.gov, a service of the U.S. National Institutes of Health, Available at http://clinicaltrials.gov/ct2/ results?term = resveratrol. Accessed March 2008
- Chow H H, Cai Y, Hakim I A, Crowell J A, Shahi F, Brooks C A, Dorr R T, Hara Y, Alberts D S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res 2003; 9: 3312–3319
- Fujiki H. Two stages of cancer prevention with green tea. J Cancer Res Clin 1999; 125: 589–597
- Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev 2003; 23: 519–534
- Pezzuto J. Natural product cancer chemopreventive agents. Recent Advances in Phytochemistry, J Arnason, R Mata, J Romeo. Plenum Press, New York 1995; Vol 29: 19–45
- Pezzuto J, Song L, Lee S, Shamon L, Mata-Greenwood E, Jang J, Jeong H-J, Pisha E, Mehta R, Kinghorn A. Bioassay methods useful for activity-guided isolation of natural product cancer chemopreventive agents. Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas, K Hostettmann, M Gupta, A Marston. Harwood Academic Publishers, ChurSwitzerland 1998; 81–110
- Kinghorn A, Su B-N, Lee D, Gu J-Q, Pezzuto J. Cancer chemopreventive agents discovered by activity-guided fractionation: An update. Curr Org Chem 2003; 7: 213–226
- Pezzuto J, Kosmeder J, Park E, Lee S, Cuendet M, Gills J, Bhat K, Grubjesic S, Park H-S, Mata-Greenwood E, Tan Y, Yu R, Lantvit D, Kinghorn A. Characterization of natural product chemopreventive agents. Cancer Chemoprevention Volume 2: Strategies for Cancer Chemoprevention, G Kelloff, E Hawk, C Sigman. Humana Press Inc., Totowa, NJ 2005; 3–37
- Loub W, Farnsworth N, Soejarto D, Quinn M. NAPRALERT: Computer handling of natural product research data. J Chem Inf Computer Sci 1985; 25: 99–103
- Kosmeder J W, II, Pezzuto J M. Intermediate biomarkers. Intermediate biomarkers 2001; 106: 31–61
- Crowell J, Holmes C. Agent identification and preclinical testing. Cancer Treat Res 2001; 106: 1–30
- Mehta R, Moon R. Characterization of effective chemopreventive agents in mammary gland in vitro. using an initiation-promotion protocol. Anticancer Res 1991; 11: 593–596
- Mehta R G, Bhat K P, Hawthorne M E, Kopelovich L, Mehta R R, Christov K, Kelloff G J, Steele V E, Pezzuto J M. Induction of atypical ductal hyperplasia in mouse mammary gland organ culture. Induction of atypical ductal hyperplasia in mouse mammary gland organ culture 2001; 93: 1103–1106
- Shamon L, Pezzuto J. Assessment of antimutagenic activity with Salmonella typhimurium. strain TM677. Methods Cell Sci 1997; 19: 57–62
- Lee S K, Mbwambo Z H, Chung H, Luyengi L, Gamez E J, Mehta R G, Kinghorn A D, Pezzuto J M. Evaluation of the antioxidant potential of natural products. Evaluation of the antioxidant potential of natural products 1998; 1: 35–46
- Song L L, Kosmeder J W, 2nd, Lee S K, Gerhauser C, Lantvit D, Moon R C, Moriarty R M, Pezzuto J M. Cancer chemopreventive activity mediated by 4′-bromoflavone, a potent inducer of phase II detoxification enzymes. Cancer chemopreventive activity mediated by 4′-bromoflavone, a potent inducer of phase II detoxification enzymes 1999; 59: 578–585
- Kang Y H, Pezzuto J M. Induction of quinone reductase as a primary screen for natural product anticarcinogens. Induction of quinone reductase as a primary screen for natural product anticarcinogens 2004; 382: 380–414
- Gerhäuser C, Mar W, Lee S, Suh N, Luo Y, Kosmeder J, Moriarty R, Luyengi L, Kinghorn A, Fong H, Mehta R, Constantinou A, Moon R, Pezzuto J. Rotenoids mediate potent chemopreventive activity through transcriptional regulation of ornithine decarboxylase. Nature Med 1995; 1: 260–266
- Mbwambo Z H, Lee S K, Mshiu E N, Pezzuto J M, Kinghorn A D. Constituents from the stem wood of Euphorbia quinquecostata. with phorbol dibutyrate receptor-binding inhibitory activity. J Nat Prod 1996; 59: 1051–1055
- Jang M, Cai L, Udeani G O, Slowing K V, Thomas C F, Beecher C W, Fong H H, Farnsworth N R, Kinghorn A D, Mehta R G, Moon R C, Pezzuto J M. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes 1997; 275: 218–220
- El S, ayed K A, Hamann M T, Waddling C A, Jensen C, Lee S K, Dunstan C A, Pezzuto J M. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation. Structurally novel bioconversion products of the marine natural product sarcophine effectively inhibit JB6 cell transformation 1998; 63: 7449–7455
- Suh N, Luyengi L, Fong H H, Kinghorn A D, Pezzuto J M. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model. Discovery of natural product chemopreventive agents utilizing HL-60 cell differentiation as a model 1995; 15: 233–239
- Pisha E, Pezzuto J. Cell-based assay for the determination of estrogenic and anti-estrogenic activity. Meth Cell Sci 1997; 19: 37–43
- Jeong H J, Shin Y G, Kim I H, Pezzuto J M. Inhibition of aromatase activity by flavonoids. Inhibition of aromatase activity by flavonoids 1999; 22: 309–312
- Chang L C, Gills J J, Bhat K P, Luyengi L, Farnsworth N R, Pezzuto J M, Kinghorn A D. Activity-guided isolation of constituents of Cerbera manghas. with antiproliferative and antiestrogenic activities. Bioorg Med Chem Lett 2000; 10: 2431–2434
- Gamez E J, Luyengi L, Lee S K, Zhu L F, Zhou B N, Fong H H, Pezzuto J M, Kinghorn A D. Antioxidant flavonoid glycosides from Daphniphyllum calycinum.. J Nat Prod 1998; 61: 706–70
- Su B N, Park E J, Nikolic D, Santarsiero B D, Mesecar A D, Vigo J S, Graham J G, Cabieses F, van Breemen R B, Fong H H, Farnsworth N R, Pezzuto J M, Kinghorn A D. Activity-guided isolation of novel norwithanolides from Deprea subtriflora. with potential cancer chemopreventive activity. J Org Chem 2003; 68: 2350–2361
- Gu J Q, Park E J, Luyengi L, Hawthorne M E, Mehta R G, Farnsworth N R, Pezzuto J M, Kinghorn A D. Constituents of Eugenia sandwicensis. with potential cancer chemopreventive activity. Phytochemistry 2001; 58: 121–127
- Johnson B, Nikolic D, van Breemen R. Applications of pulsed ultrafiltration-mass spectrometry. Mass Spectrom Rev 2002; 21: 76–86
- Kinghorn A D, Su B N, Jang D S, Chang L C, Lee D, Gu J Q, Carcache-Blanco E J, Pawlus A D, Lee S K, Park E J, Cuendet M, Gills J J, Bhat K, Park H S, Mata-Greenwood E, Song L L, Jang M, Pezzuto J M. Natural inhibitors of carcinogenesis. Natural inhibitors of carcinogenesis 2004; 70: 691–705
- Su B N, Park E J, Nikolic D, Schunke Vigo J, Graham J G, Cabieses F, van Breemen R B, Fong H H, Farnsworth N R, Pezzuto J M, Kinghorn A D. Isolation and characterization of miscellaneous secondary metabolites of Deprea subtriflora.. J Nat Prod 2003; 66: 1089–1093
- Lee D, Cuendet M, Vigo J S, Graham J G, Cabieses F, Fong H H, Pezzuto J M, Kinghorn A D. A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata.. Org Lett 2001; 3: 2169–2171
- Lee D, Park E J, Cuendet M, Axelrod F, Chavez P I, Fong H H, Pezzuto J M, Kinghorn A D. Cyclooxygenase-inhibitory and antioxidant constituents of the aerial parts of Antirhea acutata.. Bioorg Med Chem Lett 2001; 11: 1565–1568
- Mehta R G, Liu J, Constantinou A, Thomas C F, Hawthorne M, You M, Gerhäuser C, Pezzuto J M, Moon R C, Moriarty R M. Cancer chemopreventive activity of brassinin, a phytoalexin from cabbage. Cancer chemopreventive activity of brassinin, a phytoalexin from cabbage 1995; 16: 399–404
- Gerhäuser C, Lee S K, Kosmeder J W, Moriarty R M, Hamel E, Mehta R G, Moon R C, Pezzuto J M. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent. Regulation of ornithine decarboxylase induction by deguelin, a natural product cancer chemopreventive agent 1997; 57: 3429–3435
- Udeani G O, Gerhauser C, Thomas C F, Moon R C, Kosmeder J W, Kinghorn A D, Moriarty R M, Pezzuto J M. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid. Cancer chemopreventive activity mediated by deguelin, a naturally occurring rotenoid 1997; 57: 3424–3428
- Lee H Y, Suh Y A, Kosmeder J W, Pezzuto J M, Hong W K, Kurie J M. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells. Deguelin-induced inhibition of cyclooxygenase-2 expression in human bronchial epithelial cells 2004; 10: 1074–1079
- Murillo G, Hirschelman W H, Ito A, Moriarty R M, Kinghorn A D, Pezzuto J M, Mehta G. Zapotin, a phytochemical present in a Mexican fruit, prevents colon carcinogenesis. Nutr Cancer 2007; 57: 28–37
- Mata-Greenwood E, Cuendet M, Sher D, Gustin D, Stock W, Pezzuto J M. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc. Brusatol-mediated induction of leukemic cell differentiation and G(1) arrest is associated with down-regulation of c-myc 2002; 16: 2275–2284
- Cuendet M, Christov K, Lantvit D D, Deng Y, Hedayat S, Helson L, McChesney J D, Pezzuto J M. Multiple myeloma regression mediated by bruceantin. Multiple myeloma regression mediated by bruceantin 2004; 10: 1170–1179
- Lee D, Bhat K P, Fong H H, Farnsworth N R, Pezzuto J M, Kinghorn A D. Aromatase inhibitors from Broussonetia papyrifera.. J Nat Prod 2001; 64: 1286–1293
- Zhang Y, Kensler T W, Cho C G, Posner G H, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci USA 1994; 91: 3147–3150
- Gerhäuser C, You M, Liu J, Moriarty R M, Hawthorne M, Mehta R G, Moon R C, Pezzuto J M. Cancer chemopreventive potential of sulforamate, a novel analogue of sulforaphane that induces phase 2 drug-metabolizing enzymes. Cancer chemopreventive potential of sulforamate, a novel analogue of sulforaphane that induces phase 2 drug-metabolizing enzymes 1997; 57: 272–278
- Kosmeder J W, II, Hirschelman W H, Song L S, Park E J, Tan Y, Yu R, Hawthorne M, Mehta R G, Grubbs C J, Lubet R A, Moriarty R M, Pezzuto J M. Cancer chemopreventive activity of oxomate, a monofunctional inducer of Phase II detoxification enzymes. 224th American Chemical Society National Meeting. Boston, Massachusetts. , August 18–22, 2002
- Stewart J R, Artime M C, O'Brian C A. Resveratrol: a candidate nutritional substance for prostate cancer prevention. J Nutr 2003; 133: 2440S–2443S
- Creasy L, Coffee M. Phytoalexin production potential of grape berries. J Am Soc Hortic Sci 1988; 113: 230–234
- Schwekendiek A, Pfeffer G, Kindl H. Pine stilbene synthase cDNA, a tool for probing environmental stress. FEBS Lett 1992; 301: 41–44
- Jeandet P, Douillet-Breuil A C, Bessis R, Debord S, Sbaghi M, Adrian M. Phytoalexins from the Vitaceae.: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 2002; 50: 2731–2741
- Montero C, Cristescu S, Jimenez J, Orea J, Hekkert StL, Harren F, Urena A G. trans.-Resveratrol and grape disease resistance, a dynamic study by high-resolution laser-based techniques. Plant Physiol 2003; 131: 129–138
- Hawksworth D. Micological research news. Mycol Res 2003; 107: 769–770
- Siemann G, Creasy L. Concentration of the phytoalexin resveratrol in wine. Am J Ecol Viticul 1992; 43: 49–52
- Cantos E, Garcia-Viguera C, Pascual-Teresa S, Tomas-Barberan F. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J Agric Food Chem 2000; 48: 4604–4612
- Careri M, Corradini C, Elviri L, Nicoletti I, Zagnoni I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. J Agric Food Chem 2003; 51: 5226–5231
- Nonomura S, Kanagawa H, Makimoto A. Chemical constituents of polygonaceous plants. I. Studies on the components of Ko-jo-kon (Polygonum cuspidatum. SIEB et ZUCC). Yukugaku Zasshi 1963; 83: 983–988
- Hain R, Reif H J, Krause E, Langebartels R, Kindl H, Vornam B, Wiese W, Schmelzer E, Schreier P H, Stocker R H, Stenzel K. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993; 361: 153–156
- Paiva N. International Molecular Farming Conference. Engineering Resveratrol Accumulation into Alfalfa and Other Food Plants. London, OntarioCanada 1999; 134
- Proceedings of a Conference Exploring the Power of Phytochemicals. Research Advances on Grape Compounds, J Pezzuto, V Steele. Swets and Zeitlinger, LisseThe Netherlands 1998, (A supplement of Pharm Biol.)
- Bhat K PL, Kosmeder J W, 2nd, Pezzuto J M. Biological effects of resveratrol. Biological effects of resveratrol 2001; 3: 1041–1064
- Subbaramaiah K, Chung W J, Michaluart P, Telang N, Tanabe T, Inoue H, Jang M, Pezzuto J M, Dannenberg A J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells 1998; 273: 21875–21882
- Jang M, Pezzuto J M. Cancer chemopreventive activity of resveratrol. Cancer chemopreventive activity of resveratrol 1999; 25: 65–77
- Bhat K P, Pezzuto J M. Cancer chemopreventive activity of resveratrol. Cancer chemopreventive activity of resveratrol 2002; 957: 210–229
- Pezzuto J, Kondratyuk T, Shalaev E. Cancer chemoprevention by wine polyphenols and resveratrol. Carcinogenic and Anticarcinogenic Food Components, W Baer-Dubowska, A Bartoszek, D Malejka-Giganti. CRC Press, Boca Raton, FL 2006; 239–282
- Bagchi D. Resveratrol and Human Health. McGraw Hill, Columbus, OH 2000
- Aggarwal B, Shishodia S. Resveratrol in Health and Disease. Taylor & Francis, Boca Raton, FL 2006
- Szumilo J. Resveratrol—evaluation of anticancer activity. Pol Merkur Lekarski 2006; 20: 362–364
- Baur J A, Sinclair D A. Therapeutic potential of resveratrol: The in vivo. evidence. Nat Rev Drug Discov 2006; 5: 493–506
- Delmas D, Lancon A, Colin D, Jannin B, Latruffe N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr Drug Targets 2006; 7: 423–442
- Aggarwal B B, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 2006; 71: 1397–1421
- Trosko J E. The role of stem cells and gap junctions as targets for cancer chemoprevention and chemotherapy. Biomed Pharmacother 2005; 59(Suppl 2)S326–331
- Yance D R, Jr., Sagar S M. Targeting angiogenesis with integrative cancer therapies. Targeting angiogenesis with integrative cancer therapies 2006; 5: 9–29
- Baliga M S, Katiyar S K. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Chemoprevention of photocarcinogenesis by selected dietary botanicals 2006; 5: 243–253
- Anderson L M. Cancer biology and hormesis: Comments on Calabrese. Crit Rev Toxicol 2005; 35: 583–586
- Garg A K, Buchholz T A, Aggarwal B B. Chemosensitization and radiosensitization of tumors by plant polyphenols. Chemosensitization and radiosensitization of tumors by plant polyphenols 2005; 7: 1630–1647
- Ovesna Z, Horvathova-Kozics K. Structure-activity relationship of trans.-resveratrol and its analogues. Neoplasma 2005; 52: 450–455
- Trosko J E, Chang C C, Upham B L, Tai M H. The role of human adult stem cells and cell-cell communication in cancer chemoprevention and chemotherapy strategies. The role of human adult stem cells and cell-cell communication in cancer chemoprevention and chemotherapy strategies 2005; 591: 187–197
- Kundu J K, Surh Y J. Molecular basis of chemoprevention by resveratrol: NF-κ B and AP-1 as potential targets. Molecular basis of chemoprevention by resveratrol: NF-κ B and AP-1 as potential targets 2004; 555: 65–80
- Bode A M, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res 2004; 555: 33–51
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J Nutr Biochem 2005; 16: 449–466
- Shimizu M, Weinstein I B. Modulation of signal transduction by tea catechins and related phytochemicals. Modulation of signal transduction by tea catechins and related phytochemicals 2005; 591: 147–160
- Morris B J. A forkhead in the road to longevity: The molecular basis of lifespan becomes clearer. J Hypertens 2005; 23: 1285–1309
- Tisdale M J. The ubiquitin-proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol 2005; 3: 209–217
- Stopper H, Schmitt E, Kobras K. Genotoxicity of phytoestrogens. Mutat Res 2005; 574: 139–155
- Guastalla J P, Bachelot T, Ray-Coquard I. Cyclooxygenase 2 and breast cancer. From biological concepts to clinical trials. Bull Cancer 2004; 91(Suppl 2)S99–108
- de l a, Lastra C A, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: Mechanisms and clinical implications. Mol Nutr Food Res 2005; 49: 405–430
- Ulrich S, Wolter F, Stein J M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis 2005; 49: 452–461
- Ray A. Cancer preventive role of selected dietary factors. Indian J Cancer 2005; 42: 15–24
- Kimura Y. New anticancer agents: In vitro. and in vivo. evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo 2005; 19: 37–60
- Pervaiz S. Chemotherapeutic potential of the chemopreventive phytoalexin resveratrol. Drug Resist Updat 2004; 7: 333–344
- Le C, orre L, Chalabi N, Delort L, Bignon Y J, Bernard-Gallon DJ. Resveratrol and breast cancer chemoprevention: Molecular mechanisms. Mol Nutr Food Res 2005; 49: 462–471
- Granados-Soto V. Pleiotropic effects of resveratrol. Drug News Perspect 2003; 16: 299–307
- Atten M J, Godoy-Romero E, Attar B M, Milson T, Zopel M, Holian O. Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs 2005; 23: 111–119
- Gescher A. Polyphenolic phytochemicals versus non-steroidal anti-inflammatory drugs: Which are better cancer chemopreventive agents?. J Chemother 16 Suppl 2004; 4: 3–6
- Manson M M, Farmer P B, Gescher A, Steward W P. Innovative agents in cancer prevention. Innovative agents in cancer prevention 2005; 166: 257–275
- Oak M H, El Bedoui J, Schini-Kerth V B. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem 2005; 16: 1–8
- Choi S M, Lee B M. An alternative mode of action of endocrine-disrupting chemicals and chemoprevention. An alternative mode of action of endocrine-disrupting chemicals and chemoprevention 2004; 7: 451–463
- Wolter F, Ulrich S, Stein J. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in colorectal cancer: Key role of polyamines?. J Nutr 2004; 134: 3219–3222
- Aggarwal B B, Bhardwaj A, Aggarwal R S, Seeram N P, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res 2004; 24: 2783–2840
- Dorai T, Aggarwal B B. Role of chemopreventive agents in cancer therapy. Role of chemopreventive agents in cancer therapy 2004; 215: 129–140
- Aggarwal B B, Takada Y, Oommen O V. From chemoprevention to chemotherapy: Common targets and common goals. From chemoprevention to chemotherapy: Common targets and common goals 2004; 13: 1327–1338
- Jannin B, Menzel M, Berlot J P, Delmas D, Lancon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol 2004; 68: 1113–1118
- Simopoulos A P. The traditional diet of Greece and cancer. Eur J Cancer Prev 2004; 13: 219–230
- Park J W, Clark O H. Redifferentiation therapy for thyroid cancer. Redifferentiation therapy for thyroid cancer 2004; 84: 921–943
- Ho S M. Estrogens and anti-estrogens: Key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem 2004; 91: 491–503
- Li Y, Shin Y G, Yu C, Kosmeder J W, Hirschelman W H, Pezzuto J M, van Breemen R B. Increasing the throughput and productivity of Caco-2 cell permeability assays using liquid chromatography-mass spectrometry: Application to resveratrol absorption and metabolism. Increasing the throughput and productivity of Caco-2 cell permeability assays using liquid chromatography-mass spectrometry: Application to resveratrol absorption and metabolism 2003; 6: 757–767
- Gescher A J, Steward W P. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: A conundrum. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: A conundrum 2003; 12: 953–957
- Bianchini F, Vainio H. Wine and resveratrol: Mechanisms of cancer prevention?. Eur J Cancer Prev 2003; 12: 417–425
- Bhavnani B R. Estrogens and menopause: Pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. J Steroid Biochem Mol Biol 2003; 85: 473–482
- Frank G C. From sandwiches to center stage. Peanuts pack a powerful nutritional punch. Adv Nurse Pract 2003; 11: 85–87, 95
- Lopez-Velez M, Martinez-Martinez F, Del Valle-Ribes C. The study of phenolic compounds as natural antioxidants in wine. Crit Rev Food Sci Nutr 2003; 43: 233–244
- Kimura Y. Pharmacological studies on resveratrol. Methods Find Exp Clin Pharmacol 2003; 25: 297–310
- Aziz M H, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: In vitro. and in vivo. studies and the underlying mechanisms (review). Int J Oncol 2003; 23: 17–28
- Corpet D E, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev 2003; 12: 391–400
- Cal C, Garban H, Jazirehi A, Yeh C, Mizutani Y, Bonavida B. Resveratrol and cancer: Chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr Med Chem Anticancer Agents 2003; 3: 77–93
- Safe S H, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environ Health Perspect 110 Suppl 2002; 6: 925–929
- Guengerich F P, Chun Y J, Kim D, Gillam E M, Shimada T. Cytochrome P450 1B1: A target for inhibition in anticarcinogenesis strategies. Mutat Res 523– 2003; 524: 173–182
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res 523– 2003; 524: 145–150
- Roemer K, Mahyar-Roemer M. The basis for the chemopreventive action of resveratrol. Drugs Today (Barc) 2002; 38: 571–580
- Thampatty B P, Rosenkranz H S. Structural concepts in cancer prevention. Structural concepts in cancer prevention 2002; 11 Suppl 2: S76–85
- Kris-Etherton P M, Hecker K D, Bonanome A, Coval S M, Binkoski A E, Hilpert K F, Griel A E, Etherton T D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer 2002; 113(Suppl 9B)71S–88S
- Park E J, Pezzuto J M. Botanicals in cancer chemoprevention. Botanicals in cancer chemoprevention 2002; 21: 231–255
- Ratan H L, Steward W P, Gescher A J, Mellon J K. Resveratrol—a prostate cancer chemopreventive agent?. Urol Oncol 2002; 7: 223–227
- Mehta R G, Pezzuto J M. Discovery of cancer preventive agents from natural products: From plants to prevention. Discovery of cancer preventive agents from natural products: From plants to prevention 2002; 4: 478–486
- Culig Z, Klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer 2002; 9: 155–170
- Tsan M F, White J E, Maheshwari J G, Chikkappa G. Anti-leukemia effect of resveratrol. Leuk Lymphoma 2002; 43: 983–987
- Milner J A, McDonald S S, Anderson D E, Greenwald P. Molecular targets for nutrients involved with cancer prevention. Nutr Cancer 2001; 41: 1–16
- Savouret J F, Quesne M. Resveratrol and cancer: A review. Biomed Pharmacother 2002; 56: 84–87
- Ignatowicz E, Baer-Dubowska W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol J Pharmacol 2001; 53: 557–569
- Bode A M, Dong Z. Signal transduction pathways: Targets for chemoprevention of skin cancer. Lancet Oncol 2000; 1: 181–188
- Afaq F, Adhami V M, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci 2002; 7: d784–792
- Stierum R, Burgemeister R, van Helvoort A, Peijnenburg A, Schutze K, Seidelin M, Vang O, van Ommen B. Functional food ingredients against colorectal cancer. An example project integrating functional genomics, nutrition and health. Nutr Metab Cardiovasc Dis 2001; 11: 94–98
- Safe S H, Pallaroni L, Yoon K, Gaido K, Ross S, Saville B, McDonnell D. Toxicology of environmental estrogens. Reprod Fertil Dev 2001; 13: 307–315
- Wargovich M J. Colon cancer chemoprevention with ginseng and other botanicals. J Korean Med Sci 2001; 16(Suppl)S81–86
- Simopoulos A P. The Mediterranean diets: What is so special about the diet of Greece? The scientific evidence. J Nutr 2001; 131: 3065S–3073S
- Surh Y J, Chun K S, Cha H H, Han S S, Keum Y S, Park K K, Lee S S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κ B activation. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κ B activation 2001; 481: 243–268
- Kong A N, Yu R, Hebbar V, Chen C, Owuor E, Hu R, Ee R, Mandlekar S. Signal transduction events elicited by cancer prevention compounds. Mutat Res 480– 2001; 481: 231–241
- Ciolino H P, Yeh G C. The effects of resveratrol on CYP1A1 expression and aryl hydrocarbon receptor function in vitro.. Adv Exp Med Biol 2001; 492: 183–193
- Soleas G J, Diamandis E P, Goldberg D M. The world of resveratrol. The world of resveratrol 2001; 492: 159–182
- Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis 2001; 22: 1111–1117
- Pervaiz S. Resveratrol—from the bottle to the bedside?. Leuk Lymphoma 2001; 40: 491–498
- Yang C S, Landau J M, Huang M T, Newmark H L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Inhibition of carcinogenesis by dietary polyphenolic compounds 2001; 21: 381–406
- Olas B, Wachowicz B. Biological activity of resveratrol. Postepy Hig Med Dosw 2001; 55: 71–79
- Ahmad N, Katiyar S K, Mukhtar H. Antioxidants in chemoprevention of skin cancer. Curr Probl Dermatol 2001; 29: 128–139
- Dong Z. Effects of food factors on signal transduction pathways. Biofactors 2000; 12: 17–28
- Cuendet M, Pezzuto J M. The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. The role of cyclooxygenase and lipoxygenase in cancer chemoprevention 2000; 17: 109–157
- Hadi S M, Asad S F, Singh S, Ahmad A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000; 50: 167–171
- Huber J. Phytoestrogens and SERMS, alternatives to classical hormone therapy?. Ther Umsch 2000; 57: 651–654
- Weisburger J H. Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem Toxicol 1999; 37: 943–948
- Lin J K, Tsai S H. Chemoprevention of cancer and cardiovascular disease by resveratrol. Chemoprevention of cancer and cardiovascular disease by resveratrol 1999; 23: 99–106
- Calabrese G. Nonalcoholic compounds of wine: The phytoestrogen resveratrol and moderate red wine consumption during menopause. Drugs Exp Clin Res 1999; 25: 111–114
- Tredici G, Miloso M, Nicolini G, Galbiati S, Cavaletti G, Bertelli A. Resveratrol, map kinases and neuronal cells: Might wine be a neuroprotectant?. Drugs Exp Clin Res 1999; 25: 99–103
- Soleas G J, Diamandis E P, Goldberg D M. Wine as a biological fluid: History, production, and role in disease prevention. Wine as a biological fluid: History, production, and role in disease prevention 1997; 11: 287–313
- Russo G L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol 2007; 74: 533–544
- Malemud C J. Inhibitors of stress-activated protein/mitogen-activated protein kinase pathways. Curr Opin Pharmacol 2007; 7: 339–343
- Pettit G R, Grealish M P, Jung M K, Hamel E, Pettit R K, Chapuis J C, Schmidt J M. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate 2002; 45: 2534–2542
- Kim S, Ko H, Park J E, Jung S, Lee S K, Chun Y J. Design, synthesis, and discovery of novel trans.-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem 2002; 45: 160–164
- Thakkar K, Geahlen R L, Cushman M. Synthesis and protein-tyrosine kinase inhibitory activity of polyhydroxylated stilbene analogues of piceatannol. J Med Chem 1993; 36: 2950–2955
- Cushman M, Nagarathnam D, Gopal D, He H M, Lin C M, Hamel E. Synthesis and evaluation of analogues of Z.)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethene as potential cytotoxic and antimitotic agents. J Med Chem 1992; 35: 2293–2306
- Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M. Synthesis and biological evaluation of resveratrol and analogues as apoptosis-inducing agents. J Med Chem 2003; 46: 3546–3554
- Szewczuk L M, Penning T M. Mechanism-based inactivation of COX-1 by red wine m.-hydroquinones: A structure-activity relationship study. J Nat Prod 2004; 67: 1777–1782
- Wang Z, Hsieh T C, Zhang Z, Ma Y, Wu J M. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography. Identification and purification of resveratrol targeting proteins using immobilized resveratrol affinity chromatography 2004; 323: 743–749
- Leonard S S, Xia C, Jiang B H, Stinefelt B, Klandorf H, Harris G K, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun 2003; 309: 1017–1026
- Potter G A, Patterson L H, Wanogho E, Perry P J, Butler P C, Ijaz T, Ruparelia K C, Lamb J H, Farmer P B, Stanley L A, Burke M D. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1. The cancer preventative agent resveratrol is converted to the anticancer agent piceatannol by the cytochrome P450 enzyme CYP1B1 2002; 86: 774–778
- Kim Y M, Yun J, Lee C K, Lee H, Min K R, Kim Y. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 2002; 277: 16340–16344
- Burkhardt S, Reiter R J, Tan D X, Hardeland R, Cabrera J, Karbownik M. DNA oxidatively damaged by chromium(III) and H2O2 is protected by the antioxidants melatonin, N.1-acetyl-N.2-formyl-5-methoxykynuramine, resveratrol and uric acid. Int J Biochem Cell Biol 2001; 33: 775–783
- de S a, nti C, Pietrabissa A, Mosca F, Pacifici G M. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver 2000; 30: 1047–1054
- Stewart J R, Christman K L, O'Brian C A. Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases: Inhibition of protein kinase D but not protein kinase C isozyme autophosphorylation. Biochem Pharmacol 2000; 60: 1355–1359
- de S, anti C, Pietrabissa A, Spisni R, Mosca F, Pacifici G M. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum 2000; 30: 609–617
- Garcia-Garcia J, Micol V, de Godos A, Gomez-Fernandez J C. The cancer chemopreventive agent resveratrol is incorporated into model membranes and inhibits protein kinase C alpha activity. Arch Biochem Biophys 1999; 372: 382–388
- Stewart J R, Ward N E, Ioannides C G, O'Brian C A. Resveratrol preferentially inhibits protein kinase C-catalyzed phosphorylation of a cofactor-independent, arginine-rich protein substrate by a novel mechanism. Biochemistry 1999; 38: 13244–13251
- Chun Y J, Kim M Y, Guengerich F P. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Resveratrol is a selective human cytochrome P450 1A1 inhibitor 1999; 262: 20–24
- Feng L, Jin J, Zhang L F, Yan T, Tao W Y. Analysis of the resveratrol-binding protein using phage-displayed random peptide library. Acta Biochim Biophys Sin (Shanghai) 2006; 38: 342–348
- Jo J Y, Gonzalez de Mejia E, Lila M A. Catalytic inhibition of human DNA topoisomerase II by interactions of grape cell culture polyphenols. Catalytic inhibition of human DNA topoisomerase II by interactions of grape cell culture polyphenols 2006; 54: 2083–2087
- Fukuhara K, Nagakawa M, Nakanishi I, Ohkubo K, Imai K, Urano S, Fukuzumi S, Ozawa T, Ikota N, Mochizuki M, Miyata N, Okuda H. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II). Bioorg Med Chem 2006; 14: 1437–1443
- Srivastava R, Ratheesh A, Gude R K, Rao K V, Panda D, Subrahmanyam G. Resveratrol inhibits type II phosphatidylinositol 4-kinase: A key component in pathways of phosphoinositide turn over. Biochem Pharmacol 2005; 70: 1048–1055
- Ahmad A, Syed F A, Singh S, Hadi S M. Prooxidant activity of resveratrol in the presence of copper ions: Mutagenicity in plasmid DNA. Prooxidant activity of resveratrol in the presence of copper ions: Mutagenicity in plasmid DNA 2005; 159: 1–12
- Onuki J, Almeida E A, Medeiros M H, Di Mascio P. Inhibition of 5-aminolevulinic acid-induced DNA damage by melatonin, N1-acetyl-N2-formyl-5-methoxykynuramine, quercetin or resveratrol. J Pineal Res 2005; 38: 107–115
- Lu Z, Zhang Y, Liu H, Yuan J, Zheng Z, Zou G. Transport of a cancer chemopreventive polyphenol, resveratrol: Interaction with serum albumin and hemoglobin. J Fluoresc 2007; 17: 580–587
- Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J 2007; 406: 511–518
- Waffo-Teguo P, Hawthorne M E, Cuendet M, Merillon J M, Kinghorn A D, Pezzuto J M, Mehta R G. Potential cancer-chemopreventive activities of wine stilbenoids and flavans extracted from grape (Vitis vinifera.) cell cultures. Nutr Cancer 2001; 40: 173–179
- Mutoh M, Takahashi M, Fukuda K, Matsushima-Hibiya Y, Mutoh H, Sugimura T, Wakabayashi K. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis 2000; 21: 959–963
- Castello L, Tessitore L. Resveratrol inhibits cell cycle progression in U937 cells. Oncol Rep 2005; 13: 133–137
- Narayanan N K, Narayanan B A, Nixon D W. Resveratrol-induced cell growth inhibition and apoptosis is associated with modulation of phosphoglycerate mutase B in human prostate cancer cells: Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mass spectrometry evaluation. Resveratrol-induced cell growth inhibition and apoptosis is associated with modulation of phosphoglycerate mutase B in human prostate cancer cells: Two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mass spectrometry evaluation 2004; 28: 443–452
- Shih A, Zhang S, Cao H J, Boswell S, Wu Y H, Tang H Y, Lennartz M R, Davis F B, Davis P J, Lin H Y. Inhibitory effect of epidermal growth factor on resveratrol-induced apoptosis in prostate cancer cells is mediated by protein kinase C-alpha. Inhibitory effect of epidermal growth factor on resveratrol-induced apoptosis in prostate cancer cells is mediated by protein kinase C-alpha 2004; 3: 1355–1364
- Scifo C, Cardile V, Russo A, Consoli R, Vancheri C, Capasso F, Vanella A, Renis M. Resveratrol and propolis as necrosis or apoptosis inducers in human prostate carcinoma cells. Oncol Res 2004; 14: 415–426
- Delmas D, Rebe C, Micheau O, Athias A, Gambert P, Grazide S, Laurent G, Latruffe N, Solary E. Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 2004; 23: 8979–8986
- Ma X, Tian X, Huang X, Yan F, Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca(2+) and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem 2007; 302: 99–109
- Wang S, Wang X, Yan J, Xie X, Fan F, Zhou X, Han L, Chen J. Resveratrol inhibits proliferation of cultured rat cardiac fibroblasts: Correlated with NO-cGMP signaling pathway. Eur J Pharmacol 2007; 567: 26–35
- Su J L, Yang C Y, Zhao M, Kuo M L, Yen M L. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol 2007; 282: 19385–19398
- Nonn L, Duong D, Peehl D M. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells 2007; 28: 1188–1196
- Kim Y A, Kim G Y, Park K Y, Choi Y H. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia 2007; 10: 218–224
- Benitez D A, Pozo-Guisado E, Clementi M, Castellon E, Fernandez-Salguero P M. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br J Cancer 2007; 96: 1595–1604
- Lee E J, Min H Y, Joo Park H, Chung H J, Kim S, Nam Han Y, Lee S K. G2/M cell cycle arrest and induction of apoptosis by a stilbenoid, 3,4,5-trimethoxy-4′-bromo-cis.-stilbene, in human lung cancer cells. Life Sci 2004; 75: 2829–2839
- Bianco N R, Chaplin L J, Montano M M. Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage. Differential induction of quinone reductase by phytoestrogens and protection against oestrogen-induced DNA damage 2005; 385: 279–287
- Yuan H, Pan Y, Young C Y. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells 2004; 213: 155–163
- Cao Z, Fang J, Xia C, Shi X, Jiang B H. trans.-3,4,5′-Trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res 2004; 10: 5253–5263
- Shi T, Liou L S, Sadhukhan P, Duan Z H, Novick A C, Hissong J G, Almasan A, DiDonato J A. Effects of resveratrol on gene expression in renal cell carcinoma. Effects of resveratrol on gene expression in renal cell carcinoma 2004; 3: 882–888
- Fulda S, Debatin K M. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol. Sensitization for anticancer drug-induced apoptosis by the chemopreventive agent resveratrol 2004; 23: 6702–6711
- Hyun J Y, Chun Y S, Kim T Y, Kim H L, Kim M S, Park J W. Hypoxia-inducible factor 1alpha-mediated resistance to phenolic anticancer. Hypoxia-inducible factor 1alpha-mediated resistance to phenolic anticancer 2004; 50: 119–126
- Schneider Y, Fischer B, Coelho D, Roussi S, Gosse F, Bischoff P, Raul F. (Z.)-3,5,4′-Tri-O.-methyl-resveratrol, induces apoptosis in human lymphoblastoid cells independently of their p53 status. Cancer Lett 2004; 211: 155–161
- Quiney C, Dauzonne D, Kern C, Fourneron J D, Izard J C, Mohammad R M, Kolb J P, Billard C. Flavones and polyphenols inhibit the NO pathway during apoptosis of leukemia B-cells. Leuk Res 2004; 28: 851–861
- Zhang S, Cao H J, Davis F B, Tang H Y, Davis P J, Lin H Y. Estrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells. Estrogen inhibits resveratrol-induced post-translational modification of p53 and apoptosis in breast cancer cells 2004; 91: 178–185
- Liu J, Wang Q, Wu D C, Wang X W, Sun Y, Chen X Y, Zhang K L, Li H. Differential regulation of CYP1A1 and CYP1B1 expression in resveratrol-treated human medulloblastoma cells. Neurosci Lett 2004; 363: 257–261
- Laux M T, Aregullin M, Berry J P, Flanders J A, Rodriguez E. Identification of a p53-dependent pathway in the induction of apoptosis of human breast cancer cells by the natural product, resveratrol. J Altern Complement Med 2004; 10: 235–239
- Berge G, Ovrebo S, Botnen I V, Hewer A, Phillips D H, Haugen A, Mollerup S. Resveratrol inhibits benzo[a.]pyrene-DNA adduct formation in human bronchial epithelial cells. Br J Cancer 2004; 91: 333–338
- Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res 2004; 24: 987–998
- Le C, orre L, Fustier P, Chalabi N, Bignon Y J, Bernard-Gallon D. Effects of resveratrol on the expression of a panel of genes interacting with the BRCA1 oncosuppressor in human breast cell lines. Clin Chim Acta 2004; 344: 115–121
- Jeong W S, Kim I W, Hu R, Kong A N. Modulatory properties of various natural chemopreventive agents on the activation of NF-κ B signaling pathway. Modulatory properties of various natural chemopreventive agents on the activation of NF-κ B signaling pathway 2004; 21: 661–670
- Jeong W S, Kim I W, Hu R, Kong A N. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line 2004; 21: 649–660
- Baatout S, Derradji H, Jacquet P, Ooms D, Michaux A, Mergeay M. Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int J Mol Med 2004; 13: 895–902
- Feng Y H, Zhu Y N, Liu J, Ren Y X, Xu J Y, Yang Y F, Li X Y, Zou J P. Differential regulation of resveratrol on lipopolysacchride-stimulated human macrophages with or without IFN-gamma pre-priming. Differential regulation of resveratrol on lipopolysacchride-stimulated human macrophages with or without IFN-gamma pre-priming 2004; 4: 713–720
- Gehm B D, Levenson A S, Liu H, Lee E J, Amundsen B M, Cushman M, Jordan V C, Jameson J L. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: Role of AF-1 and AF-2. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: Role of AF-1 and AF-2 2004; 88: 223–234
- Cooray H C, Janvilisri T, van Veen H W, Hladky S B, Barrand M A. Interaction of the breast cancer resistance protein with plant polyphenols. Interaction of the breast cancer resistance protein with plant polyphenols 2004; 317: 269–275
- Lancon A, Delma D, Osman H, Thenot J P, Jannin B, Latruffe N. Human hepatic cell uptake of resveratrol: Involvement of both passive diffusion and carrier-mediated process. Biochem Biophys Res Commun 2004; 316: 1132–1137
- Gao S, Liu G Z, Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate 2004; 59: 214–225
- Cheung C Y, Chen J, Chang T K. Evaluation of a real-time polymerase chain reaction method for the quantification of CYP1B1 gene expression in MCF-7 human breast carcinoma cells. Evaluation of a real-time polymerase chain reaction method for the quantification of CYP1B1 gene expression in MCF-7 human breast carcinoma cells 2004; 49: 97–104
- Ahmad K A, Clement M V, Hanif I M, Pervaiz S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res 2004; 64: 1452–1459
- Pozo-Guisado E, Lorenzo-Benayas M J, Fernandez-Salguero P M. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: Relevance in cell proliferation. Int J Cancer 2004; 109: 167–173
- Jazirehi A R, Bonavida B. Resveratrol modifies the expression of apoptotic regulatory proteins and sensitizes non-Hodgkin's lymphoma and multiple myeloma cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 2004; 3: 71–84
- Opipari A W, Jr, Tan L, Boitano A E, Sorenson D R, Aurora A, Liu J R. Resveratrol-induced autophagocytosis in ovarian cancer cells. Resveratrol-induced autophagocytosis in ovarian cancer cells 2004; 64: 696–703
- Stewart J R, O'Brian C A. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs 2004; 22: 107–117
- Fulda S, Debatin K M. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol 2004; 64: 337–346
- Kim Y A, Choi B T, Lee Y T, Park D I, Rhee S H, Park K Y, Choi Y H. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells 2004; 11: 441–446
- Carraway R E, Hassan S, Cochrane D E. Polyphenolic antioxidants mimic the effects of 1,4-dihydropyridines on neurotensin receptor function in PC3 cells. Polyphenolic antioxidants mimic the effects of 1,4-dihydropyridines on neurotensin receptor function in PC3 cells 2004; 309: 92–101
- Woo J H, Lim J H, Kim Y H, Suh S I, Min D S, Chang J S, Lee Y H, Park J W, Kwon T K. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction. Resveratrol inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting JNK and PKC delta signal transduction 2004; 23: 1845–1853
- Sala G, Minutolo F, Macchia M, Sacchi N, Ghidoni R. Resveratrol structure and ceramide-associated growth inhibition in prostate cancer cells. Drugs Exp Clin Res 2003; 29: 263–269
- Bruno R, Ghisolfi L, Priulla M, Nicolin A, Bertelli A. Wine and tumors: Study of resveratrol. Drugs Exp Clin Res 2003; 29: 257–261
- Cardile V, Scifo C, Russo A, Falsaperla M, Morgia G, Motta M, Renis M, Imbriani E, Silvestre G. Involvement of HSP70 in resveratrol-induced apoptosis of human prostate cancer. Anticancer Res 2003; 23: 4921–4926
- Kim Y A, Rhee S H, Park K Y, Choi Y H. Antiproliferative effect of resveratrol in human prostate carcinoma cells. Antiproliferative effect of resveratrol in human prostate carcinoma cells 2003; 6: 273–280
- Kang J H, Park Y H, Choi S W, Yang E K, Lee W J. Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp Mol Med 2003; 35: 467–474
- Kubota T, Uemura Y, Kobayashi M, Taguchi H. Combined effects of resveratrol and paclitaxel on lung cancer cells. Anticancer Res 2003; 23: 4039–4046
- Wang Q, Li H, Wang X W, Wu D C, Chen X Y, Liu J. Resveratrol promotes differentiation and induces Fas-independent apoptosis of human medulloblastoma cells. Neurosci Lett 2003; 351: 83–86
- Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo. ceramide signaling. FASEB J 2003; 17: 2339–2341
- Kaneuchi M, Sasaki M, Tanaka Y, Yamamoto R, Sakuragi N, Dahiya R. Resveratrol suppresses growth of Ishikawa cells through down-regulation of EGF. Int J Oncol 2003; 23: 1167–1172
- Kim Y A, Lee W H, Choi T H, Rhee S H, Park K Y, Choi Y H. Involvement of p21WAF1/CIP1, pRB, Bax and NF-κ B in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int J Oncol 2003; 23: 1143–1149
- Schneider Y, Chabert P, Stutzmann J, Coelho D, Fougerousse A, Gosse F, Launay J F, Brouillard R, Raul F. Resveratrol analog (Z.)-3,5,4′-trimethoxystilbene is a potent anti-mitotic drug inhibiting tubulin polymerization. Int J Cancer 2003; 107: 189–196
- Delmas D, Rebe C, Lacour S, Filomenko R, Athias A, Gambert P, Cherkaoui-Malki M, Jannin B, Dubrez-Daloz L, Latruffe N, Solary E. Resveratrol-induced apoptosis is associated with Fas redistribution in the rafts and the formation of a death-inducing signaling complex in colon cancer cells. J Biol Chem 2003; 278: 41482–41490
- Ito T, Akao Y, Yi H, Ohguchi K, Matsumoto K, Tanaka T, Iinuma M, Nozawa Y. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis 2003; 24: 1489–1497
- Fustier P, Le Corre L, Chalabi N, Vissac-Sabatier C, Communal Y, Bignon Y J, Bernard-Gallon D J. Resveratrol increases BRCA1 and BRCA2 mRNA expression in breast tumour cell lines. Br J Cancer 2003; 89: 168–172
- El-Mowafy A M, Alkhalaf M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: A novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis 2003; 24: 869–873
- Bernhard D, Schwaiger W, Crazzolara R, Tinhofer I, Kofler R, Csordas A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett 2003; 195: 193–199
- Kim S, Min S Y, Lee S K, Cho W J. Comparative molecular field analysis study of stilbene derivatives active against A549 lung carcinoma. Chem Pharm Bull (Tokyo) 2003; 51: 516–521
- Wietzke J A, Welsh J. Phytoestrogen regulation of a Vitamin D3 receptor promoter and 1,25-dihydroxyvitamin D3 actions in human breast cancer cells. J Steroid Biochem Mol Biol 2003; 84: 149–157
- Estrov Z, Shishodia S, Faderl S, Harris D, Van Q, Kantarjian H M, Talpaz M, Aggarwal B B. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-κ B, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-κ B, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells 2003; 102: 987–995
- She Q B, Ma W Y, Wang M, Kaji A, Ho C T, Dong Z. Inhibition of cell transformation by resveratrol and its derivatives: Differential effects and mechanisms involved. Oncogene 2003; 22: 2143–2150
- Wolter F, Turchanowa L, Stein J. Resveratrol-induced modification of polyamine metabolism is accompanied by induction of c-Fos. Carcinogenesis 2003; 24: 469–474
- Liang Y C, Tsai S H, Chen L, Lin-Shiau S Y, Lin J K. Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Resveratrol-induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells 2003; 65: 1053–1060
- Zhou H B, Yan Y, Sun Y N, Zhu J R. Resveratrol induces apoptosis in human esophageal carcinoma cells. Resveratrol induces apoptosis in human esophageal carcinoma cells 2003; 9: 408–411
- Yang S H, Kim J S, Oh T J, Kim M S, Lee S W, Woo S K, Cho H S, Choi Y H, Kim Y H, Rha S Y, Chung H C, An S W. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray 2003; 22: 741–750
- Levenson A S, Gehm B D, Pearce S T, Horiguchi J, Simons L A, Ward J E, 3rd, Jameson J L, Jordan V C. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha 2003; 104: 587–596
- Narayanan B A, Narayanan N K, Re G G, Nixon D W. Differential expression of genes induced by resveratrol in LNCaP cells: P53-Mediated molecular targets. Differential expression of genes induced by resveratrol in LNCaP cells: P53-Mediated molecular targets 2003; 104: 204–212
- Niles R M, McFarland M, Weimer M B, Redkar A, Fu Y M, Meadows G G. Resveratrol is a potent inducer of apoptosis in human melanoma cells. Resveratrol is a potent inducer of apoptosis in human melanoma cells 2003; 190: 157–163
- Nicolini G, Rigolio R, Scuteri A, Miloso M, Saccomanno D, Cavaletti G, Tredici G. Effect of trans.-resveratrol on signal transduction pathways involved in paclitaxel-induced apoptosis in human neuroblastoma SH-SY5Y cells. Neurochem Int 2003; 42: 419–429
- Hayashibara T, Yamada Y, Nakayama S, Harasawa H, Tsuruda K, Sugahara K, Miyanishi T, Kamihira S, Tomonaga M, Maita T. Resveratrol induces downregulation in survivin expression and apoptosis in HTLV-1-infected cell lines: A prospective agent for adult T cell leukemia chemotherapy. Nutr Cancer 2002; 44: 193–201
- Roy M, Chakraborty S, Siddiqi M, Bhattacharya R K. Induction of apoptosis in tumor cells by natural phenolic compounds. Induction of apoptosis in tumor cells by natural phenolic compounds 2002; 3: 61–67
- Billard C, Izard J C, Roman V, Kern C, Mathiot C, Mentz F, Kolb J P. Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes 2002; 43: 1991–2002
- Latruffe N, Delmas D, Jannin B, Cherkaoui Malki M, Passilly-Degrace P, Berlot J P. Molecular analysis on the chemopreventive properties of resveratrol, a plant polyphenol microcomponent. Molecular analysis on the chemopreventive properties of resveratrol, a plant polyphenol microcomponent 2002; 10: 755–760
- Schmitt E, Lehmann L, Metzler M, Stopper H. Hormonal and genotoxic activity of resveratrol. Toxicol Lett 2002; 136: 133–142
- Brownson D M, Azios N G, Fuqua B K, Dharmawardhane S F, Mabry T J. Flavonoid effects relevant to cancer. Flavonoid effects relevant to cancer 2002; 132: 3482S–3489S
- Ding X Z, Adrian T E. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells. Resveratrol inhibits proliferation and induces apoptosis in human pancreatic cancer cells 2002; 25: e71–76
- Kuo P L, Chiang L C, Lin C C. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Resveratrol-induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells 2002; 72: 23–34
- Pozo-Guisado E, Alvarez-Barrientos A, Mulero-Navarro S, Santiago-Josefat B, Fernandez-Salguero P M. The antiproliferative activity of resveratrol results in apoptosis in MCF-7 but not in MDA-MB-231 human breast cancer cells: cell-specific alteration of the cell cycle. Biochem Pharmacol 2002; 64: 1375–1386
- Mahyar-Roemer M, Kohler H, Roemer K. Role of Bax in resveratrol-induced apoptosis of colorectal carcinoma cells. BMC Cancer 2002; 2: 27–35
- Hsieh T, Halicka D, Lu X, Kunicki J, Guo J, Darzynkiewicz Z, Wu J. Effects of resveratrol on the G0-G1 transition and cell cycle progression of mitogenically stimulated human lymphocytes. Biochem Biophys Res Commun 2002; 297: 1311–1317
- Melzig M F, Escher F. Induction of neutral endopeptidase and angiotensin-converting enzyme activity of SK-N-SH cells in vitro. by quercetin and resveratrol. Pharmazie 2002; 57: 556–558
- Morris G Z, Williams R L, Elliott M S, Beebe S J. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells 2002; 52: 319–329
- Dubuisson J G, Dyess D L, Gaubatz J W. Resveratrol modulates human mammary epithelial cell O.-acetyltransferase, sulfotransferase, and kinase activation of the heterocyclic amine carcinogen N.-hydroxy-PhIP. Cancer Lett 2002; 182: 27–32
- Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, Dupouy M, Thiolat D, Kolb J P, Marit G, Reiffers J, Mossalayi M D. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells 2002; 23: 1327–1333
- Kim H J, Chang E J, Bae S J, Shim S M, Park H D, Rhee C H, Park J H, Choi S W. Cytotoxic and antimutagenic stilbenes from seeds of Paeonia lactiflora.. Arch Pharm Res 2002; 25: 293–299
- Lin H Y, Shih A, Davis F B, Tang H Y, Martino L J, Bennett J A, Davis P J. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line 2002; 168: 748–755
- Delmas D, Passilly-Degrace P, Jannin B, Cherkaoui Malki M, Latruffe N. Resveratrol, a chemopreventive agent, disrupts the cell cycle control of human SW480 colorectal tumor cells. Int J Mol Med 2002; 10: 193–199
- Wolter F, Stein J. Resveratrol enhances the differentiation induced by butyrate in Caco-2 colon cancer cells. J Nutr 2002; 132: 2082–2086
- Asou H, Koshizuka K, Kyo T, Takata N, Kamada N, Koeffier H P. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias. Resveratrol, a natural product derived from grapes, is a new inducer of differentiation in human myeloid leukemias 2002; 75: 528–533
- Roman V, Billard C, Kern C, Ferry-Dumazet H, Izard J C, Mohammad R, Mossalayi D M, Kolb J P. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia 2002; 117: 842–851
- Rimando A M, Cuendet M, Desmarchelier C, Mehta R G, Pezzuto J M, Duke S O. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol. Cancer chemopreventive and antioxidant activities of pterostilbene, a naturally occurring analogue of resveratrol 2002; 50: 3453–3457
- Narayanan B A, Narayanan N K, Stoner G D, Bullock B P. Interactive gene expression pattern in prostate cancer cells exposed to phenolic antioxidants. Interactive gene expression pattern in prostate cancer cells exposed to phenolic antioxidants 2002; 70: 1821–1839
- Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack E R, Reddy G P. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis 2002; 62: 2488–2492
- Holian O, Wahid S, Atten M J, Attar B M. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide. Inhibition of gastric cancer cell proliferation by resveratrol: Role of nitric oxide 2002; 282: G809–816
- She Q B, Huang C, Zhang Y, Dong Z. Involvement of c-jun NH2-terminal kinases in resveratrol-induced activation of p53 and apoptosis. Mol Carcinog 2002; 33: 244–250
- Joe A K, Liu H, Suzui M, Vural M E, Xiao D, Weinstein I B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines 2002; 8: 893–903
- Shih A, Davis F B, Lin H Y, Davis P J. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK-and p53-dependent mechanism. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK-and p53-dependent mechanism 2002; 87: 1223–1232
- Lee S H, Ryu S Y, Kim H B, Kim M Y, Chun Y J. Induction of apoptosis by 3,4′-dimethoxy-5-hydroxystilbene in human promyeloid leukemic HL-60 cells. Induction of apoptosis by 3,4′-dimethoxy-5-hydroxystilbene in human promyeloid leukemic HL-60 cells 2002; 68: 123–127
- Pendurthi U R, Meng F, Mackman N, Rao L V. Mechanism of resveratrol-mediated suppression of tissue factor gene expression. Mechanism of resveratrol-mediated suppression of tissue factor gene expression 2002; 87: 155–162
- Sun Z J, Pan C E, Liu H S, Wang G J. Anti-hepatoma activity of resveratrol in vitro.. World J Gastroenterol 2002; 8: 79–81
- Ito T, Akao Y, Tanaka T, Iinuma M, Nozawa Y. Vaticanol C, a novel resveratrol tetramer, inhibits cell growth through induction of apoptosis in colon cancer cell lines. Biol Pharm Bull 2002; 25: 147–148
- Wolter F, Clausnitzer A, Akoglu B, Stein J. Piceatannol, a natural analog of resveratrol, inhibits progression through the S phase of the cell cycle in colorectal cancer cell lines. J Nutr 2002; 132: 298–302
- Zoberi I, Bradbury C M, Curry H A, Bisht K S, Goswami P C, Roti Roti J L, Gius D. Radiosensitizing and anti-proliferative effects of resveratrol in two human cervical tumor cell lines. Cancer Lett 2002; 175: 165–173
- Serrero G, Lu R. Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid Redox Signal 2001; 3: 969–979
- Heo Y H, Kim S, Park J E, Jeong L S, Lee S K. Induction of quinone reductase activity by stilbene analogs in mouse Hepa 1c1c7 cells. Induction of quinone reductase activity by stilbene analogs in mouse Hepa 1c1c7 cells 2001; 24: 597–600
- Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer 2001; 94: 615–622
- Atten M J, Attar B M, Milson T, Holian O. Resveratrol-induced inactivation of human gastric adenocarcinoma cells through a protein kinase C-mediated mechanism. Biochem Pharmacol 2001; 62: 1423–1432
- Nam K A, Kim S, Heo Y H, Lee S K. Resveratrol analog, 3,5,2′,4′-tetramethoxy-trans.-stilbene, potentiates the inhibition of cell growth and induces apoptosis in human cancer cells. Arch Pharm Res 2001; 24: 441–445
- Wieder T, Prokop A, Bagci B, Essmann F, Bernicke D, Schulze-Osthoff K, Dorken B, Schmalz H G, Daniel P T, Henze G. Piceatannol, a hydroxylated analog of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia 2001; 15: 1735–1742
- Adhami V M, Afaq F, Ahmad N. Involvement of the retinoblastoma (pRb)-E2F/DP pathway during antiproliferative effects of resveratrol in human epidermoid carcinoma (A431) cells. Biochem Biophys Res Commun 2001; 288: 579–585
- Lee J E, Safe S. Involvement of a post-transcriptional mechanism in the inhibition of CYP1A1 expression by resveratrol in breast cancer cells. Biochem Pharmacol 2001; 62: 1113–1124
- Park J W, Choi Y J, Suh S I, Baek W K, Suh M H, Jin I N, Min D S, Woo J H, Chang J S, Passaniti A, Lee Y H, Kwon T K. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity 2001; 22: 1633–1639
- Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf F I, Cittadini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res 2001; 496: 171–180
- Bhat K P, Pezzuto J M. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells 2001; 61: 6137–6144
- Wolter F, Akoglu B, Clausnitzer A, Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr 2001; 131: 2197–2203
- Dorrie J, Gerauer H, Wachter Y, Zunino S J. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells. Resveratrol induces extensive apoptosis by depolarizing mitochondrial membranes and activating caspase-9 in acute lymphoblastic leukemia cells 2001; 61: 4731–4739
- De L, edinghen V, Monvoisin A, Neaud V, Krisa S, Payrastre B, Bedin C, Desmouliere A, Bioulac-Sage P, Rosenbaum J. trans.-Resveratrol, a grapevine-derived polyphenol, blocks hepatocyte growth factor-induced invasion of hepatocellular carcinoma cells. Int J Oncol 2001; 19: 83–88
- Kozuki Y, Miura Y, Yagasaki K. Resveratrol suppresses hepatoma cell invasion independently of its anti-proliferative action. Cancer Lett 2001; 167: 151–156
- Ahmad N, Adhami V M, Afaq F, Feyes D K, Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res 2001; 7: 1466–1473
- Nakagawa H, Kiyozuka Y, Uemura Y, Senzaki H, Shikata N, Hioki K, Tsubura A. Resveratrol inhibits human breast cancer cell growth and may mitigate the effect of linoleic acid, a potent breast cancer cell stimulator. J Cancer Res Clin Oncol 2001; 127: 258–264
- Mollerup S, Ovrebo S, Haugen A. Lung carcinogenesis: Resveratrol modulates the expression of genes involved in the metabolism of PAH in human bronchial epithelial cells. Int J Cancer 2001; 92: 18–25
- Nicolini G, Rigolio R, Miloso M, Bertelli A A, Tredici G. Anti-apoptotic effect of trans.-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett 2001; 302: 41–44
- She Q B, Bode A M, Ma W Y, Chen N Y, Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res 2001; 61: 1604–1610
- Lu J, Ho C H, Ghai G, Chen K Y. Resveratrol analog, 3,4,5,4′-tetrahydroxystilbene, differentially induces pro-apoptotic p53/Bax gene expression and inhibits the growth of transformed cells but not their normal counterparts. Resveratrol analog, 3,4,5,4′-tetrahydroxystilbene, differentially induces pro-apoptotic p53/Bax gene expression and inhibits the growth of transformed cells but not their normal counterparts 2001; 22: 321–328
- Park J W, Choi Y J, Jang M A, Lee Y S, Jun D Y, Suh S I, Baek W K, Suh M H, Jin I N, Kwon T K. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells 2001; 163: 43–49
- Kampa M, Hatzoglou A, Notas G, Damianaki A, Bakogeorgou E, Gemetzi C, Kouroumalis E, Martin P M, Castanas E. Wine antioxidant polyphenols inhibit the proliferation of human prostate cancer cell lines. Nutr Cancer 2000; 37: 223–233
- Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner M J, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acute leukemia cells. Cell Death Differ 2000; 7: 834–842
- Bowers J L, Tyulmenkov V V, Jernigan S C, Klinge C M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta 2000; 141: 3657–3667
- Nielsen M, Ruch R J, Vang O. Resveratrol reverses tumor-promoter-induced inhibition of gap-junctional intercellular communication. Biochem Biophys Res Commun 2000; 275: 804–809
- Schneider Y, Vincent F, Duranton B, Badolo L, Gosse F, Bergmann C, Seiler N, Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett 2000; 158: 85–91
- Holmes-McNary M, Baldwin A S, Jr. Chemopreventive properties of trans.-resveratrol are associated with inhibition of activation of the Iκ B kinase. Cancer Res 2000; 60: 3477–3483
- Delmas D, Jannin B, Cherkaoui Malki M, Latruffe N. Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol Rep 2000; 7: 847–852
- Tsan M F, White J E, Maheshwari J G, Bremner T A, Sacco J. Resveratrol induces Fas signalling-independent apoptosis in THP-1 human monocytic leukaemia cells. Br J Haematol 2000; 109: 405–412
- Hsieh T C, Wu J M. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism. Grape-derived chemopreventive agent resveratrol decreases prostate-specific antigen (PSA) expression in LNCaP cells by an androgen receptor (AR)-independent mechanism 2000; 20: 225–228
- Godichaud S, Krisa S, Couronne B, Dubuisson L, Merillon J M, Desmouliere A, Rosenbaum J. Deactivation of cultured human liver myofibroblasts by trans.-resveratrol, a grapevine-derived polyphenol. Hepatology 2000; 31: 922–931
- Elattar T M, Virji A S. The effect of red wine and its components on growth and proliferation of human oral squamous carcinoma cells. The effect of red wine and its components on growth and proliferation of human oral squamous carcinoma cells 1999; 19: 5407–5414
- Mitchell S H, Zhu W, Young C Y. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells 1999; 59: 5892–5895
- Ulsperger E, Hamilton G, Raderer M, Baumgartner G, Hejna M, Hoffmann O, Mallinger R. Resveratrol pretreatment desensitizes AHTO-7 human osteoblasts to growth stimulation in response to carcinoma cell supernatants. Int J Oncol 1999; 15: 955–959
- Surh Y J, Hurh Y J, Kang J Y, Lee E, Kong G, Lee S J. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells 1999; 140: 1–10
- Hsieh T C, Burfeind P, Laud K, Backer J M, Traganos F, Darzynkiewicz Z, Wu J M. Cell cycle effects and control of gene expression by resveratrol in human breast carcinoma cell lines with different metastatic potentials. Cell cycle effects and control of gene expression by resveratrol in human breast carcinoma cell lines with different metastatic potentials 1999; 15: 245–252
- Hsieh T C, Wu J M. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines 1999; 249: 109–115
- Miloso M, Bertelli A A, Nicolini G, Tredici G. Resveratrol-induced activation of the mitogen-activated protein kinases, ERK1 and ERK2, in human neuroblastoma SH-SY5Y cells. Neurosci Lett 1999; 264: 141–144
- Lu R, Serrero G. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol 1999; 179: 297–304
- Elattar T M, Virji A S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation 1999; 10: 187–193
- Huang C, Ma W Y, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis 1999; 20: 237–242
- Clement M V, Hirpara J L, Chawdhury S H, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998; 92: 996–1002
- Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett 1998; 421: 277–279
- Jang M, Pezzuto J. Resveratrol blocks eicosanoid production and chemically induced cellular transformation: Implications for cancer chemoprevention. Pharm Biol 1998; 36: 28–34
- Gehm B D, McAndrews J M, Chien P Y, Jameson J L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor 1997; 94: 14138–14143
- Ulrich S, Loitsch S M, Rau O, von Knethen A, Brune B, Schubert-Zsilavecz M, Stein J M. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism. Peroxisome proliferator-activated receptor gamma as a molecular target of resveratrol-induced modulation of polyamine metabolism 2006; 66: 7348–7354
- Michels G, Watjen W, Weber N, Niering P, Chovolou Y, Kampkotter A, Proksch P, Kahl R. Resveratrol induces apoptotic cell death in rat H4IIE hepatoma cells but necrosis in C6 glioma cells. Toxicology 2006; 225: 173–182
- Alkhalaf M, Jaffal S. Potent antiproliferative effects of resveratrol on human osteosarcoma SJSA1 cells: Novel cellular mechanisms involving the ERKs/p53 cascade. Free Radic Biol Med 2006; 41: 318–325
- Lin H Y, Lansing L, Merillon J M, Davis F B, Tang H Y, Shih A, Vitrac X, Krisa S, Keating T, Cao H J, Bergh J, Quackenbush S, Davis P J. Integrin alphaVbeta3 contains a receptor site for resveratrol. Integrin alphaVbeta3 contains a receptor site for resveratrol 2006; 20: 1742–1744
- Seve M, Chimienti F, Devergnas S, Aouffen M, Douki T, Chantegrel J, Cadet J, Favier A. Resveratrol enhances UVA-induced DNA damage in HaCaT human keratinocytes. Med Chem 2005; 1: 629–633
- Kim A L, Zhu Y, Zhu H, Han L, Kopelovich L, Bickers D R, Athar M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol 2006; 15: 538–546
- Aziz M H, Nihal M, Fu V X, Jarrard D F, Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol Cancer Ther 2006; 5: 1335–1341
- Roberti M, Pizzirani D, Recanatini M, Simoni D, Grimaudo S, Di Cristina A, Abbadessa V, Gebbia N, Tolomeo M. Identification of a terphenyl derivative that blocks the cell cycle in the G0-G1 phase and induces differentiation in leukemia cells. J Med Chem 2006; 49: 3012–3018
- Kim Y A, Lim S Y, Rhee S H, Park K Y, Kim C H, Choi B T, Lee S J, Park Y M, Choi Y H. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells 2006; 17: 1069–1075
- Ma X D, Yan F, Ma A D, Wang H J. Resveratrol induces HepG2 cell apoptosis by depolarizing mitochondrial membrane. Resveratrol induces HepG2 cell apoptosis by depolarizing mitochondrial membrane 2006; 26: 406–408, 413
- Yoo K M, Kim S, Moon B K, Kim S S, Kim K T, Kim S Y, Choi S Y. Potent inhibitory effects of resveratrol derivatives on progression of prostate cancer cells. Arch Pharm (Weinheim) 2006; 339: 238–241
- Mohan J, Gandhi A A, Bhavya B C, Rashmi R, Karunagaran D, Indu R, Santhoshkumar T R. Caspase-2 triggers Bax-Bak-dependent and-independent cell death in colon cancer cells treated with resveratrol. Caspase-2 triggers Bax-Bak-dependent and-independent cell death in colon cancer cells treated with resveratrol 2006; 281: 17599–17611
- Wang Y, Lee K W, Chan F L, Chen S, Leung L K. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells 2006; 92: 71–77
- Balestrieri C, Felice F, Piacente S, Pizza C, Montoro P, Oleszek W, Visciano V, Balestrieri M L. Relative effects of phenolic constituents from Yucca schidigera. Roezl. bark on Kaposi's sarcoma cell proliferation, migration, and PAF synthesis. Biochem Pharmacol 2006; 71: 1479–1487
- Scifo C, Milasi A, Guarnera A, Sinatra F, Renis M. Resveratrol and propolis extract: An insight into the morphological and molecular changes induced in DU145 cells. Oncol Res 2006; 15: 409–421
- Pohland T, Wagner S, Mahyar-Roemer M, Roemer K. Bax and Bak are the critical complementary effectors of colorectal cancer cell apoptosis by chemopreventive resveratrol. Anticancer Drugs 2006; 17: 471–478
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos A S, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther 2006; 5: 621–629
- Wen X, Walle T. Cytochrome P450 1B1, a novel chemopreventive target for benzo[a.]pyrene-initiated human esophageal cancer. Cancer Lett 2007; 246: 109–114
- Lee S C, Chan J, Clement M V, Pervaiz S. Functional proteomics of resveratrol-induced colon cancer cell apoptosis: Caspase-6-mediated cleavage of lamin A is a major signaling loop. Proteomics 2006; 6: 2386–2394
- Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa M T, Tomas-Barberan F A, Espin J C. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities 2006; 54: 1611–1620
- Choi J K, Murillo G, Su B N, Pezzuto J M, Kinghorn A D, Mehta R G. Ixocarpalactone A isolated from the Mexican tomatillo shows potent antiproliferative and apoptotic activity in colon cancer cells. Ixocarpalactone A isolated from the Mexican tomatillo shows potent antiproliferative and apoptotic activity in colon cancer cells 2006; 273: 5714–5723
- Tsuji P A, Walle T. Inhibition of benzo[a.]pyrene-activating enzymes and DNA binding in human bronchial epithelial BEAS-2B cells by methoxylated flavonoids. Carcinogenesis 2006; 27: 1579–1585
- Lanzilli G, Fuggetta M P, Tricarico M, Cottarelli A, Serafino A, Falchetti R, Ravagnan G, Turriziani M, Adamo R, Franzese O, Bonmassar E. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro.. Int J Oncol 2006; 28: 641–648
- Shimizu T, Nakazato T, Xian M J, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem Pharmacol 2006; 71: 742–750
- Azmi A S, Bhat S H, Hanif S, Hadi S M. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties 2006; 580: 533–538
- Liao H F, Kuo C D, Yang Y C, Lin C P, Tai H C, Chen Y Y, Chen Y J. Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-κ B activation. J Radiat Res (Tokyo) 2005; 46: 387–393
- Vyas S, Asmerom Y, De Leon D D. Insulin-like growth factor II mediates resveratrol stimulatory effect on cathepsin D in breast cancer cells. Insulin-like growth factor II mediates resveratrol stimulatory effect on cathepsin D in breast cancer cells 2006; 24: 79–87
- Yang S, Irani K, Heffron S E, Jurnak F, Meyskens F L, Jr. Alterations in the expression of the apurinic/ apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther 2005; 4: 1923–1935
- Young L F, Martin K R. Time-dependent resveratrol-mediated mRNA and protein expression associated with cell cycle in WR-21 cells containing mutated human c-Ha-Ras. Time-dependent resveratrol-mediated mRNA and protein expression associated with cell cycle in WR-21 cells containing mutated human c-Ha-Ras 2006; 50: 70–77
- Garcia M, ediero J M, Ferruelo Alonso A, Paez Borda A, Lujan Galan M, Angulo Cuesta J, Chiva Robles V, Berenguer Sanchez A. Effect of polyphenols from the Mediterranean diet on proliferation and mediators of in vitro. invasiveness of the MB-49 murine bladder cancer cell line. Actas Urol Esp 2005; 29: 743–749
- Arimochi H, Morita K. High salt culture conditions suppress proliferation of rat C6 glioma cell by arresting cell-cycle progression at S-phase. J Mol Neurosci 2005; 27: 293–301
- Supornsilchai V, Svechnikov K, Seidlova-Wuttke D, Wuttke W, Soder O. Phytoestrogen resveratrol suppresses steroidogenesis by rat adrenocortical cells by inhibiting cytochrome P450 c21-hydroxylase. Horm Res 2005; 64: 280–286
- Boissy P, Andersen T L, Abdallah B M, Kassem M, Plesner T, Delaisse J M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation 2005; 65: 9943–9952
- Minutolo F, Sala G, Bagnacani A, Bertini S, Carboni I, Placanica G, Prota G, Rapposelli S, Sacchi N, Macchia M, Ghidoni R. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J Med Chem 2005; 48: 6783–6786
- Zhang Q, Tang X, Lu Q Y, Zhang Z F, Brown J, Le A D. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells 2005; 4: 1465–1474
- Zunino S J, Storms D H. Resveratrol-induced apoptosis is enhanced in acute lymphoblastic leukemia cells by modulation of the mitochondrial permeability transition pore. Resveratrol-induced apoptosis is enhanced in acute lymphoblastic leukemia cells by modulation of the mitochondrial permeability transition pore 2006; 240: 123–134
- Nifli A P, Kampa M, Alexaki V I, Notas G, Castanas E. Polyphenol interaction with the T47D human breast cancer cell line. J Dairy Res 2005; 72(Spec No)44–50
- Harris D M, Besselink E, Henning S M, Go V L, Heber D. Phytoestrogens induce differential estrogen receptor alpha-or beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005; 230: 558–568
- Katula K S, McCain J A, Radewicz A T. Relative ability of dietary compounds to modulate nuclear factor-κ B activity as assessed in a cell-based reporter system. Relative ability of dietary compounds to modulate nuclear factor-κ B activity as assessed in a cell-based reporter system 2005; 8: 269–274
- Cao Y, Wang F, Liu H Y, Fu Z D, Han R. Resveratrol induces apoptosis and differentiation in acute promyelocytic leukemia (NB4) cells. J Asian Nat Prod Res 2005; 7: 633–641
- Miller M E, Holloway A C, Foster W G. Benzo[a.]pyrene increases invasion in MDA-MB-231 breast cancer cells via increased COX-II expression and prostaglandin E2 (PGE2) output. Clin Exp Metastasis 2005; 22: 149–156
- Yang C, Wu J, Zhang R, Zhang P, Eckard J, Yusuf R, Huang X, Rossman T G, Frenkel K. Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology 2005; 213: 81–96
- Gagliano N, Moscheni C, Torri C, Magnani I, Bertelli A A, Gioia M. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and Secreted Protein Acidic and Rich in Cysteine (SPARC) on human cultured glioblastoma cells. Biomed Pharmacother 2005; 59: 359–364
- Young L F, Hantz H L, Martin K R. Resveratrol modulates gene expression associated with apoptosis, proliferation and cell cycle in cells with mutated human c-Ha-Ras, but does not alter c-Ha-Ras mRNA or protein expression. Resveratrol modulates gene expression associated with apoptosis, proliferation and cell cycle in cells with mutated human c-Ha-Ras, but does not alter c-Ha-Ras mRNA or protein expression 2005; 16: 663–674
- Vyas S, Asmerom Y, De Leon D D. Resveratrol regulates insulin-like growth factor-II in breast cancer cells. Resveratrol regulates insulin-like growth factor-II in breast cancer cells 2005; 146: 4224–4233
- Chow A W, Murillo G, Yu C, van Breemen R B, Boddie A W, Pezzuto J M, Das Gupta T K, Mehta R G. Resveratrol inhibits rhabdomyosarcoma cell proliferation. Resveratrol inhibits rhabdomyosarcoma cell proliferation 2005; 14: 351–356
- Hsieh T C, Wang Z, Hamby C V, Wu J M. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53. Inhibition of melanoma cell proliferation by resveratrol is correlated with upregulation of quinone reductase 2 and p53 2005; 334: 223–230
- Kowalski J, Samojedny A, Paul M, Pietsz G, Wilczok T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774.2 macrophages. Pharmacol Rep 2005; 57: 390–394
- Tyagi A, Singh R P, Agarwal C, Siriwardana S, Sclafani R A, Agarwal R. Resveratrol causes Cdc2-tyr15 phosphorylation via ATM/ATR-Chk1/2-Cdc25C pathway as a central mechanism for S phase arrest in human ovarian carcinoma Ovcar-3 cells. Carcinogenesis 2005; 26: 1978–1987
- Azmi A S, Bhat S H, Hadi S M. Resveratrol-CuII induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett 2005; 579: 3131–3135
- Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a.]pyrene in human oral epithelial cells: Impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis 2005; 26: 1774–1781
- Bottone F G, Jr., Moon Y, Kim J S, Alston-Mills B, Ishibashi M, Eling T E. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3). The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3) 2005; 4: 693–703
- Wu M L, Li H, Wu D C, Wang X W, Chen X Y, Kong Q Y, Ma J X, Gao Y, Liu J. CYP1A1 and CYP1B1 expressions in medulloblastoma cells are AhR-independent and have no direct link with resveratrol-induced differentiation and apoptosis. Neurosci Lett 2005; 384: 33–37
- Jacobs M N, Nolan G T, Hood S R. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) 2005; 209: 123–133
- Tolomeo M, Grimaudo S, Di Cristina A, Roberti M, Pizzirani D, Meli M, Dusonchet L, Gebbia N, Abbadessa V, Crosta L, Barucchello R, Grisolia G, Invidiata F, Simoni D. Pterostilbene and 3′-hydroxypterostilbene are effective apoptosis-inducing agents in MDR and BCR-ABL-expressing leukemia cells. Int J Biochem Cell Biol 2005; 37: 1709–1726
- Matsumura A, Ghosh A, Pope G S, Darbre P D. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells 2005; 94: 431–443
- Galfi P, Jakus J, Molnar T, Neogrady S, Csordas A. Divergent effects of resveratrol, a polyphenolic phytostilbene, on free radical levels and type of cell death induced by the histone deacetylase inhibitors butyrate and trichostatin A. J Steroid Biochem Mol Biol 2005; 94: 39–47
- Waite K A, Sinden M R, Eng C. Phytoestrogen exposure elevates PTEN levels. Hum Mol Genet 2005; 14: 1457–1463
- Jiang H, Zhang L, Kuo J, Kuo K, Gautam S C, Groc L, Rodriguez A I, Koubi D, Hunter T J, Corcoran G B, Seidman M D, Levine R A. Resveratrol-induced apoptotic death in human U251 glioma cells. Resveratrol-induced apoptotic death in human U251 glioma cells 2005; 4: 554–561
- Azios N G, Dharmawardhane S F. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells 2005; 7: 128–140
- Chatterjee A, Bagchi D, Yasmin T, Stohs S J. Antimicrobial effects of antioxidants with and without clarithromycin on Helicobacter pylori.. Mol Cell Biochem 2005; 270: 125–130
- Monthakantirat O, De-Eknamkul W, Umehara K, Yoshinaga Y, Miyase T, Warashina T, Noguchi H. Phenolic constituents of the rhizomes of the Thai medicinal plant Belamcanda chinensis. with proliferative activity for two breast cancer cell lines. J Nat Prod 2005; 68: 361–364
- Andreescu S, Sadik O A, McGee D W. Effect of natural and synthetic estrogens on A549 lung cancer cells: Correlation of chemical structures with cytotoxic effects. Effect of natural and synthetic estrogens on A549 lung cancer cells: Correlation of chemical structures with cytotoxic effects 2005; 18: 466–474
- Jones S B, DePrimo S E, Whitfield M L, Brooks J D. Resveratrol-induced gene expression profiles in human prostate cancer cells. Resveratrol-induced gene expression profiles in human prostate cancer cells 2005; 14: 596–604
- Fulda S, Debatin K M. Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling. Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling 2005; 41: 786–798
- Rodrigue C M, Porteu F, Navarro N, Bruyneel E, Bracke M, Romeo P H, Gespach C, Garel M C. The cancer chemopreventive agent resveratrol induces tensin, a cell-matrix adhesion protein with signaling and antitumor activities. The cancer chemopreventive agent resveratrol induces tensin, a cell-matrix adhesion protein with signaling and antitumor activities 2005; 24: 3274–3284
- Horvath Z, Saiko P, Illmer C, Madlener S, Hoechtl T, Bauer W, Erker T, Jaeger W, Fritzer-Szekeres M, Szekeres T. Synergistic action of resveratrol, an ingredient of wine, with Ara-C and tiazofurin in HL-60 human promyelocytic leukemia cells. Exp Hematol 2005; 33: 329–335
- Pozo-Guisado E, Merino J M, Mulero-Navarro S, Lorenzo-Benayas M J, Centeno F, Alvarez-Barrientos A, Fernandez-Salguero P M. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κ B. Int J Cancer 2005; 115: 74–84
- Miura D, Miura Y, Yagasaki K. Resveratrol inhibits hepatoma cell invasion by suppressing gene expression of hepatocyte growth factor via its reactive oxygen species-scavenging property. Clin Exp Metastasis 2004; 21: 445–451
- Mertens-Talcott S U, Percival S S. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells. Ellagic acid and quercetin interact synergistically with resveratrol in the induction of apoptosis and cause transient cell cycle arrest in human leukemia cells 2005; 218: 141–151
- Lee S H, Kim J S, Yamaguchi K, Eling T E, Baek S J. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner 2005; 328: 63–69
- Rigolio R, Miloso M, Nicolini G, Villa D, Scuteri A, Simone M, Tredici G. Resveratrol interference with the cell cycle protects human neuroblastoma SH-SY5Y cell from paclitaxel-induced apoptosis. Neurochem Int 2005; 46: 205–211
- Gosslau A, Chen M, Ho C T, Chen K Y. A methoxy derivative of resveratrol analogue selectively induced activation of the mitochondrial apoptotic pathway in transformed fibroblasts. A methoxy derivative of resveratrol analogue selectively induced activation of the mitochondrial apoptotic pathway in transformed fibroblasts 2005; 92: 513–521
- Cardile V, Lombardo L, Spatafora C, Tringali C. Chemo-enzymatic synthesis and cell-growth inhibition activity of resveratrol analogues. Bioorg Chem 2005; 33: 22–33
- Wietzke J A, Ward E C, Schneider J, Welsh J. Regulation of the human vitamin D3 receptor promoter in breast cancer cells is mediated through Sp1 sites. Mol Cell Endocrinol 2005; 230: 59–68
- Awad A B, Burr A T, Fink C S. Effect of resveratrol and beta-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells. Effect of resveratrol and beta-sitosterol in combination on reactive oxygen species and prostaglandin release by PC-3 cells 2005; 72: 219–226
- Baatout S, Derradji H, Jacquet P, Mergeay M. Increased radiation sensitivity of an eosinophilic cell line following treatment with epigallocatechin-gallate, resveratrol and curcuma. Int J Mol Med 2005; 15: 337–352
- Cao Y, Fu Z D, Wang F, Liu H Y, Han R. Anti-angiogenic activity of resveratrol, a natural compound from medicinal plants. J Asian Nat Prod Res 2005; 7: 205–213
- Tsuji P A, Walle T. Benzo[a.]pyrene-induced cytochrome P450 1A and DNA binding in cultured trout hepatocytes-inhibition by plant polyphenols. Chem Biol Interact 2007; 169: 25–31
- Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, Botturi M, Ghidoni R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett 2007; 253: 124–130
- Alkhalaf M. Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur J Cancer Prev 2007; 16: 334–341
- Hibasami H, Takagi K, Ishii T, Tsujikawa M, Imai N, Honda I. Induction of apoptosis by rhapontin having stilbene moiety, a component of rhubarb (Rheum officinale. Baillon) in human stomach cancer KATO III cells. Oncol Rep 2007; 18: 347–351
- Tang F Y, Chiang E P, Sun Y C. Resveratrol inhibits heregulin-beta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. Resveratrol inhibits heregulin-beta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells 2008; 19: 287–294
- Shankar S, Siddiqui I, Srivastava R K. Molecular mechanisms of resveratrol (3,4′,5-trihydroxy-trans.-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem 2007; 304: 273–285
- Ulrich S, Huwiler A, Loitsch S, Schmidt H, Stein J M. De novo. ceramide biosynthesis is associated with resveratrol-induced inhibition of ornithine decarboxylase activity. Biochem Pharmacol 2007; 74: 281–289
- Heiss E H, Schilder Y D, Dirsch V M. Chronic treatment with resveratrol induces redox stress-and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells. Chronic treatment with resveratrol induces redox stress-and ataxia telangiectasia-mutated (ATM)-dependent senescence in p53-positive cancer cells 2007; 282: 26759–26766
- Hansen T, Seidel A, Borlak J. The environmental carcinogen 3-nitrobenzanthrone and its main metabolite 3-aminobenzanthrone enhance formation of reactive oxygen intermediates in human A549 lung epithelial cells. Toxicol Appl Pharmacol 2007; 221: 222–234
- Gunther S, Ruhe C, Derikito M G, Bose G, Sauer H, Wartenberg M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett 2007; 250: 25–35
- Dolfini E, Roncoroni L, Dogliotti E, Sala G, Erba E, Sacchi N, Ghidoni R. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett 2007; 249: 143–147
- Sakoguchi-Okada N, Takahashi-Yanaga F, Fukada K, Shiraishi F, Taba Y, Miwa Y, Morimoto S, Iida M, Sasaguri T. Celecoxib inhibits the expression of survivin via the suppression of promoter activity in human colon cancer cells. Biochem Pharmacol 2007; 73: 1318–1329
- Trincheri N F, Nicotra G, Follo C, Castino R, Isidoro C. Resveratrol induces cell death in colorectal cancer cells by a novel pathway involving lysosomal cathepsin D. Carcinogenesis 2007; 28: 922–931
- Tang X, Zhang Q, Nishitani J, Brown J, Shi S, Le A D. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells. Overexpression of human papillomavirus type 16 oncoproteins enhances hypoxia-inducible factor 1 alpha protein accumulation and vascular endothelial growth factor expression in human cervical carcinoma cells 2007; 13: 2568–2576
- Golkar L, Ding X Z, Ujiki M B, Salabat M R, Kelly D L, Scholtens D, Fought A J, Bentrem D J, Talamonti M S, Bell R H, Adrian T E. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of macrophage inhibitory cytokine-1 2007; 138: 163–169
- Clarke D M, Robilotto A T, Rhee E, VanBuskirk R G, Baust J G, Gage A A, Baust J M. Cryoablation of renal cancer: variables involved in freezing-induced cell death. Cryoablation of renal cancer: variables involved in freezing-induced cell death 2007; 6: 69–79
- Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a.]pyrene-3-sulfate. Toxicol Sci 2007; 96: 227–236
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair A S, Shishodia S, Aggarwal B B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κ B-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κ B-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells 2007; 109: 2293–2302
- Subbaramaiah K, Michaluart P, Chung W J, Tanabe T, Telang N, Dannenberg A J. Resveratrol inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Resveratrol inhibits cyclooxygenase-2 transcription in human mammary epithelial cells 1999; 889: 214–223
- Wu S L, Sun Z J, Yu L, Meng K W, Qin X L, Pan C E. Effect of resveratrol and in combination with 5-FU on murine liver cancer. Effect of resveratrol and in combination with 5-FU on murine liver cancer 2004; 10: 3048–3052
- Pezzuto J M. Resveratrol: A whiff that induces a biologically specific tsunami. Cancer Biol Ther 2004; 3: 889–890
- Kapadia G J, Azuine M A, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, Nishino H. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol Res 2002; 45: 499–505
- Soleas G J, Grass L, Josephy P D, Goldberg D M, Diamandis E P. A comparison of the anticarcinogenic properties of four red wine polyphenols. A comparison of the anticarcinogenic properties of four red wine polyphenols 2006; 39: 492–497
- Reagan-Shaw S, Afaq F, Aziz M H, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene 2004; 23: 5151–5160
- Adhami V M, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-κ B in normal human keratinocytes by resveratrol. Neoplasia 2003; 5: 74–82
- Afaq F, Adhami V M, Ahmad N. Prevention of short-term ultraviolet B radiation-mediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol 2003; 186: 28–37
- Aziz M H, Reagan-Shaw S, Wu J, Longley B J, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: Relevance to human disease?. FASEB J 2005; 19: 1193–1195
- Kim H, Hall P, Smith M, Kirk M, Prasain J K, Barnes S, Grubbs C. Chemoprevention by grape seed extract and genistein in carcinogen-induced mammary cancer in rats is diet dependent. J Nutr 2004; 134: 3445S–3452S
- Wyke S M, Russell S T, Tisdale M J. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-κ B activation. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-κ B activation 2004; 91: 1742–1750
- Crowell J A, Korytko P J, Morrissey R L, Booth T D, Levine B S. Resveratrol-associated renal toxicity. Resveratrol-associated renal toxicity 2004; 82: 614–619
- Berge G, Ovrebo S, Eilertsen E, Haugen A, Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a.]pyrene. Br J Cancer 2004; 91: 1380–1383
- Fu Z D, Cao Y, Wang K F, Xu S F, Han R. Chemopreventive effect of resveratrol to cancer. Ai Zheng 2004; 23: 869–873
- Chen Y, Tseng S H, Lai H S, Chen W J. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice 2004; 136: 57–66
- Khanduja K L, Bhardwaj A, Kaushik G. Resveratrol inhibits N.-nitrosodiethylamine-induced ornithine decarboxylase and cyclooxygenase in mice. J Nutr Sci Vitaminol (Tokyo) 2004; 50: 61–65
- Sale S, Verschoyle R D, Boocock D, Jones D J, Wilsher N, Ruparelia K C, Potter G A, Farmer P B, Steward W P, Gescher A J. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene. Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4′-tetramethoxystilbene 2004; 90: 736–744
- Ziegler C C, Rainwater L, Whelan J, McEntee M F. Dietary resveratrol does not affect intestinal tumorigenesis in Apc(Min/+) mice. Dietary resveratrol does not affect intestinal tumorigenesis in Apc(Min/+) mice 2004; 134: 5–10
- Mishima S, Matsumoto K, Futamura Y, Araki Y, Ito T, Tanaka T, Iinuma M, Nozawa Y, Akao Y. Antitumor effect of stilbenoids from Vateria indica. against allografted sarcoma S-180 in animal model. J Exp Ther Oncol 2003; 3: 283–288
- Sato M, Pei R J, Yuri T, Danbara N, Nakane Y, Tsubura A. Prepubertal resveratrol exposure accelerates N.-methyl-N.-nitrosourea-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett 2003; 202: 137–145
- Yu L, Sun Z J, Wu S L, Pan C E. Effect of resveratrol on cell cycle proteins in murine transplantable liver cancer. Effect of resveratrol on cell cycle proteins in murine transplantable liver cancer 2003; 9: 2341–2343
- Breinholt V M, Molck A M, Svendsen G W, Daneshvar B, Vinggaard A M, Poulsen M, Dragsted L O. Effects of dietary antioxidants and 2-amino-3-methylimidazo[4,5-f]-quinoline (IQ) on preneoplastic lesions and on oxidative damage, hormonal status, and detoxification capacity in the rat. Effects of dietary antioxidants and 2-amino-3-methylimidazo[4,5-f]-quinoline (IQ) on preneoplastic lesions and on oxidative damage, hormonal status, and detoxification capacity in the rat 2003; 41: 1315–1323
- Revel A, Raanani H, Younglai E, Xu J, Rogers I, Han R, Savouret J F, Casper R F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a.]pyrene. J Appl Toxicol 2003; 23: 255–261
- Liu H S, Pan C E, Yang W, Liu X M. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice 2003; 9: 1474–1476
- Miura D, Miura Y, Yagasaki K. Hypolipidemic action of dietary resveratrol, a phytoalexin in grapes and red wine, in hepatoma-bearing rats. Life Sci 2003; 73: 1393–1400
- Vitrac X, Desmouliere A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Merillon J M. Distribution of [14C]-trans.-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci 2003; 72: 2219–2233
- Li H, Cheng Y, Wang H, Sun H, Liu Y, Liu K, Peng S. Inhibition of nitrobenzene-induced DNA and hemoglobin adductions by dietary constituents. Appl Radiat Isot 2003; 58: 291–298
- Banerjee S, Bueso-Ramos C, Aggarwal B B. Suppression of 7,12-dimethylbenz(a.)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-κ B, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res 2002; 62: 4945–4954
- Li Z G, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N.-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis 2002; 23: 1531–1536
- Asensi M, Medina I, Ortega A, Carretero J, Bano M C, Obrador E, Estrela J M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Inhibition of cancer growth by resveratrol is related to its low bioavailability 2002; 33: 387–398
- Rimando A M, Nagmani R, Feller D R, Yokoyama W. Pterostilbene, a new agonist for the peroxisome proliferator-activated receptor alpha-isoform, lowers plasma lipoproteins and cholesterol in hypercholesterolemic hamsters. J Agric Food Chem 2005; 53: 3403–3407
- Mouria M, Gukovskaya A S, Jung Y, Buechler P, Hines O J, Reber H A, Pandol S J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome C release and apoptosis 2002; 98: 761–769
- Gao X, Xu Y X, Divine G, Janakiraman N, Chapman R A, Gautam S C. Disparate in vitro. and in vivo. antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr 2002; 132: 2076–2081
- Bove K, Lincoln D W, Tsan M F. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro. and in vivo.. Biochem Biophys Res Commun 2002; 291: 1001–1005
- Bhat K P, Lantvit D, Christov K, Mehta R G, Moon R C, Pezzuto J M. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models 2001; 61: 7456–7463
- Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer 2001; 39: 102–107
- Kimura Y, Okuda H. Resveratrol isolated from Polygonum cuspidatum. root prevents tumor growth and metastasis to lung and tumor-induced neovascularization in Lewis lung carcinoma-bearing mice. J Nutr 2001; 131: 1844–1849
- Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis 2000; 21: 1619–1622
- Hecht S S, Kenney P M, Wang M, Trushin N, Agarwal S, Rao A V, Upadhyaya P. Evaluation of butylated hydroxyanisole, myo-inositol, curcumin, esculetin, resveratrol and lycopene as inhibitors of benzo[a.]pyrene plus 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Lett 1999; 137: 123–130
- Carbo N, Costelli P, Baccino F M, Lopez-Soriano F J, Argiles J M. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model 1999; 254: 739–743
- Whitsett T, Carpenter M, Lamartiniere C A. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats 2006; 5: 15–25
- Valenzano D R, Cellerino A. Resveratrol and the pharmacology of aging: A new vertebrate model to validate an old molecule. Cell Cycle 2006; 5: 1027–1032
- Lee E O, Lee H J, Hwang H S, Ahn K S, Chae C, Kang K S, Lu J, Kim S H. Potent inhibition of Lewis lung cancer growth by heyneanol A from the roots of Vitis amurensis. through apoptotic and anti-angiogenic activities. Carcinogenesis 2006; 27: 2059–2069
- Rezk Y A, Balulad S S, Keller R S, Bennett J A. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: Study in human gynecologic cancer cell lines and in rodent heart. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: Study in human gynecologic cancer cell lines and in rodent heart 2006; 194: e23–26
- Chen J C, Chen Y, Lin J H, Wu J M, Tseng S H. Resveratrol suppresses angiogenesis in gliomas: Evaluation by color Doppler ultrasound. Resveratrol suppresses angiogenesis in gliomas: Evaluation by color Doppler ultrasound 2006; 26: 1237–1245
- Busquets S, Ametller E, Fuster G, Olivan M, Raab V, Argiles J M, Lopez-Soriano F J. Resveratrol, a natural diphenol, reduces metastatic growth in an experimental cancer model. Cancer Lett 2007; 245: 144–148
- Barta I, Smerak P, Polivkova Z, Sestakova H, Langova M, Turek B, Bartova J. Current trends and perspectives in nutrition and cancer prevention. Neoplasma 2006; 53: 19–25
- Walle T, Walle U K, Sedmera D, Klausner M. Benzo[a.]pyrene-induced oral carcinogenesis and chemoprevention: Studies in bioengineered human tissue. Drug Metab Dispos 2006; 34: 346–350
- Garvin S, Ollinger K, Dabrosin C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo.. Cancer Lett 2006; 231: 113–122
- Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans.-resveratrol–a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis 2006; 27: 1038–1046
- Li T, Sheng L, Fan G X, Yuan Y K, Li T. Preliminary study on anti-tumor function of resveratrol and its immunological mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2005; 21: 575–579
- Mousa S S, Mousa S S, Mousa S A. Effect of resveratrol on angiogenesis and platelet/fibrin-accelerated tumor growth in the chick chorioallantoic membrane model. Effect of resveratrol on angiogenesis and platelet/fibrin-accelerated tumor growth in the chick chorioallantoic membrane model 2005; 52: 59–65
- Hebbar V, Shen G, Hu R, Kim B R, Chen C, Korytko P J, Crowell J A, Levine B S, Kong A N. Toxicogenomics of resveratrol in rat liver. Toxicogenomics of resveratrol in rat liver 2005; 76: 2299–2314
- Provinciali M, Re F, Donnini A, Orlando F, Bartozzi B, Di Stasio G, Smorlesi A. Effect of resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int J Cancer 2005; 115: 36–45
- Sale S, Tunstall R G, Ruparelia K C, Potter G A, Steward W P, Gescher A J. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans. 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer 2005; 115: 194–201
- Zhou H B, Chen J J, Wang W X, Cai J T, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol 2005; 11: 280–284
- Porubin D, Hecht S S, Li Z Z, Gonta M, Stepanov I. Endogenous formation of N.′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric Food Chem 2007; 55: 7199–7204
- Busquets S, Fuster G, Ametller E, Olivan M, Figueras M, Costelli P, Carbo N, Argiles J M, Lopez-Soriano F J. Resveratrol does not ameliorate muscle wasting in different types of cancer cachexia models. Clin Nutr 2007; 26: 239–244
- Walle T, Hsieh F, DeLegge M H, Oatis J E, Jr., Walle U K. High absorption but very low bioavailability of oral resveratrol in humans. High absorption but very low bioavailability of oral resveratrol in humans 2004; 32: 1377–1382
- Blumberg J, Block G. The alpha-tocopherol, beta-carotene cancer prevention study in Finland. Nutr Rev 1994; 52: 242–250
- Omenn G S, Goodman G E, Thornquist M D, Balmes J, Cullen M R, Glass A, Keogh J P, Meyskens FL J r, Valanis B, Williams J J, Jr., Barnhart S, Cherniack M G, Brodkin C A, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. J Natl Cancer Inst 1996; 88: 1550–1559
- Zamora-Ros R, Urpi-Sarda M, Lamuela-Raventos R M, Estruch R, Vazquez-Agell M, Serrano-Martinez M, Jaeger W, Andres-Lacueva C. Diagnostic performance of urinary resveratrol metabolites as a biomarker of moderate wine consumption. Clin Chem 2006; 52: 1373–1380
- Lekakis J, Rallidis L S, Andreadou I, Vamvakou G, Kazantzoglou G, Magiatis P, Skaltsounis A L, Kremastinos D T. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease 2005; 12: 596–600
- Boocock D J, Faust G E, Patel K R, Schinas A M, Brown V A, Ducharme M P, Booth T D, Crowell J A, Perloff M, Gescher A J, Steward W P, Brenner D E. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent 2007; 16: 1246–1352