Abstract
In the present study, Plumbago rosea Linn. (Plumbaginaceae), one of the folk medicinal plants commonly used as an antifertility agent, was evaluated for its antifertility effect. Five successive solvent extracts, petroleum ether, chloroform, acetone, ethanol, and water extracts, of the stems of P. rosea were studied on estrous cycle at two dose levels, 200 and 400 mg/kg respectively. Of these, the acetone extract was found to be most effective in interrupting the normal estrous cycle of rats (p < 0.05) (p < 0.01). The rats exhibited prolonged diestrous stage of the estrous cycle with consequent temporary inhibition of ovulation. The antiovulatory activity was reversible on withdrawal of the extract. The effective acetone extract was further studied on estrogenic functionality in rats. The acetone extract showed significant estrogenic and antiestrogenic activity (p < 0.05) (p < 0.001). Histological studies of the uteri further confirmed the estrogenic activity of acetone extract. The results indicated the antifertility activity of stems of Plumbago rosea in female Wistar rats.
Introduction
The population explosion may have adverse economic and health effects, on the family in particular, and society in general, especially in developing countries. Accordingly, population control is of immense importance for individual and national welfare. The search for an oral contraceptive agent to control human fertility is as old as recorded history. Even though an extensive variety of synthetic contraceptive agents are available, these cannot be used constantly due to their side effects (CitationVaidya et al., 2006). Due to these side effects, an approach was pursued to identify new antifertility agents from natural sources. Numerous indigenous drugs have been described in folkloric Indian medicine for the management of various reproduction related purposes. Many plant preparations are reputed to have antifertility regulation properties and only a few have been tested for such effects. However, so far no single plant is available which can be developed into a potent antifertility agent (CitationHiremath et al., 1999).
Plumbago rosea Linn. (Plumbaginaceae), commonly known as Rakta Chitrak (CitationKritikar & Basu, 1975), grows wild and abundantly in India. Traditionally, it is used in inflammatory disorders, skin diseases (CitationDorni et al., 2006), stomachache, acidity, constipation, abdominal pain (CitationMisra et al., 2006), and as abortifacient (CitationNath & Purkayastha, 2004; CitationSarma & Mahanta, 2000; CitationDhal et al., 2000). The roots of the plant have been reported to possess antitumor (CitationDevi et al., 1994) and antiatherogenic (CitationMary et al., 2003) activities. The active constituents reported in this plant are plumbagin (CitationDevi et al., 1994) hydroxy-1,4-napthaquinone, sitosterol glycoside, fatty alcohol, and tannins (CitationAnonymous, 1985).
Throughout a literature survey it was found that the plumbagin, present in the roots of this plant, is responsible for antifertility and uterine activity (CitationRavikanth & Prakash, 1999). During our phytochemical study it was found that the stems also contain plumbagin. In this respect, we aimed to evaluate the antifertility effect of P. rosea stems. If the drug was proven to be effective, then the uprooting of this plant for abortifacient activity could be avoided.
Materials and Methods
Plant material
The stems of P. rosea were collected from Kanyakumari district, Tamil Nadu (TN) and positively identified by Dr. H.S. Chatree, Botanist, Goverment Arts and Science College, Mandsaur, Madhya Pradesh (MP). A voucher specimen (P/002/2006/BRNCOP) was deposited in the herbarium of the Department of Pharmacognosy, BRNCP, Mandsaur, for future reference.
Preparation of extracts
The stems of the plant were shade-dried and powdered. The powdered material was extracted using petroleum ether (60–80°) for 72 h and successively extracted with chloroform, acetone, ethanol, and water for 72 h each in a Soxhlet apparatus. The extract was evaporated under reduced pressure to obtain solid masses and the percentage yield of the extract was found to be 1.65%, 1.97%, 2.66%, 3.71%, and 20.50%, respectively.
Phytochemical screening
In order to determine the presence of various phytoconstituents, a preliminary phytochemical study (color reactions) with plant extracts was performed (CitationKhandelwal, 2005).
Estimation of plumbagin in various extracts
The napthaquinone identified in the petroleum ether, chloroform and acetone extracts was further confirmed by HPTLC (CitationManimaran et al., 2007). The standard (1 mg/ml) was prepared by dissolving 10 mg of plumbagin (National Chemicals, Baroda) in 10 ml of petroleum ether and 10 mg/ml sample solutions were prepared by dissolving 100 mg of the extract in 10 ml of the respective solvents. A Camag HPTLC system (Switzerland), equipped with a sample applicator Linomat IV, twin trough liner development chamber, Camag Scanner III combined with integration software CATS4.06 (Switzerland), and precoated aluminium silica gel F254 plate (Merck, Worli, Mumbai), was used for the study.
A 5 μl sample of standard plumbagin (1 mg/ml) and 5 μl of sample solutions (extracts) (10 mg/ml) were applied as a 6 mm band width at about 1 cm from the edge of a HPTLC plate using a Camag Linomat IV applicator. The solvent system was chloroform:ethyl acetate:hexane:acetic acid (10:5:5:0.3). The chromatogram was developed and scanned at 366 nm using a TLC scanner.
Animals
Female albino rats (Wistar strain weighing 150–200 g) were used for antiovulatory activity and immature female rats (Wistar strain) 21–23 days old were used for estrogenic activity. The animals were housed under standard environmental conditions of temperature (21 ± 2°C) and humidity (55 ± 10%), and a 12 h light-dark cycle. Rats were provided with standard pellet diet and water ad libitum. The animals were acclimatized to laboratory hygienic conditions for 10 days before starting the experiment. The animal study was performed in the Division of Pharmacology, B.R. Nahata College of Pharmacy, Mandsaur with due permission from the Institutional Animal Ethics Committee (Reg. No. 918/ac/05/CPCSEA).
Acute toxicity studies
The acute toxicity test of the extract was performed according to the Organization for Economic Co-operation and development guidelines No. 420 (OECD 22-08-2007). Female Wistar rats (150–180 g) were used for this study. After the pilot study, a starting dose of 2,000 mg/kg (per os) of the test samples was given to various extract groups containing 5 animals in each groups. The treated animals were monitored for 14 d for mortality and general behavior.
Antifertility activity
Antiovulatory activity
The experiment was carried out with female Wistar rats (150–200 g) as described by CitationCircosta et al. (2001). The vaginal smear of each rat was examined each morning between 9–10 a.m. for 15 days to select the animals showing regular cycles (4–5 days). The selected rats were divided into 11 groups of six animals each. The extract was administered orally for 5 days to cover one regular estrous cycle. Group I received vehicle (1% Tween 80, per os daily) and served as control. Groups II to XI received petroleum ether, chloroform, acetone, ethanol, and aqueous extracts of P. rosea stems at 200 and 400 mg/kg body weight. A vaginal smear from each animal was observed every morning between 9–10 a.m. for 5 days of treatment and during the following 15 days in the morning.
Estrogenic and antiestrogenic activity
The extract, which showed antiovulatory activity, was further studied for the estrogenic and antiestrogenic activity as described by CitationKamath and Rana (2002). Immature female Wistar strain rats, 21–23 days old, weighing between 35–45 g, were divided into 6 groups with six rats each. The first group served as control and received only vehicle (1% Tween 80). The second group received ethinyl estradiol (standard) in distilled water, 0.02 mg/kg body weight. The third and fourth groups received acetone extract of P. rosea at two dose levels, 200 and 400 mg/kg body weight, respectively. Groups five and six received ethinyl estradiol in addition to a test dose of acetone extract of the plant at the same dose. All the above treatments were given for 3 days (p.o.). On the fourth day, the rats were sacrificed by decapitation, the uteri dissected out and surrounding tissues removed. The uteri were blotted on filter paper, weighed quickly on a sensitive balance and fixed in Bouin's fluid for 24 h. The paraffin embedded tissues were cut at 6 μm and stained with hematoxylin-eosin solution for histological observations.
Statistical analysis
The data were statistically analyzed and expressed as mean ± SEM. Statistical analysis of the variance between control and experimental values was done by Student's t-test.
Results
Phytochemical screening
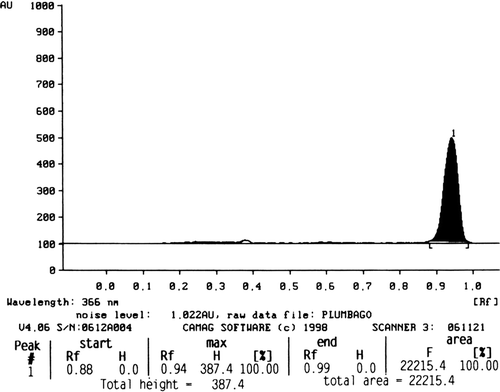
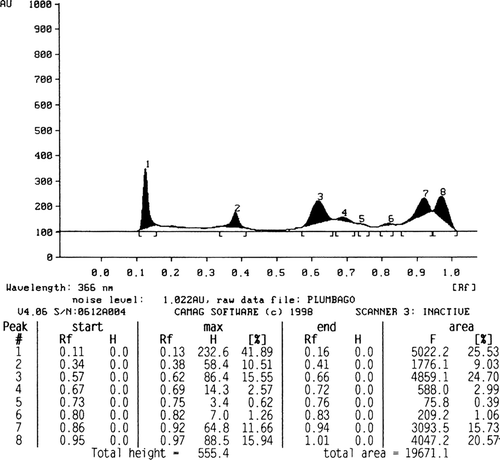
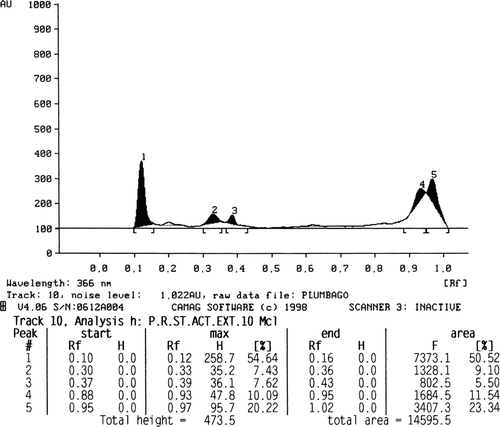
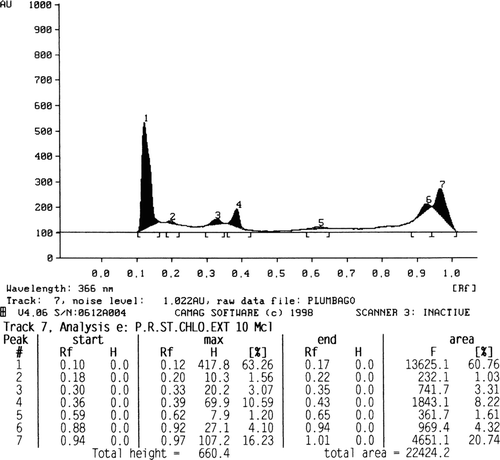
The phytochemical screening of different extracts revealed the presence of various constituents as shown in . The quantity of plumbagin was estimated by comparing the peak area of the standard () with that of the extracts. The amount of plumbagin present in petroleum ether, chloroform, and acetone extracts of stems is found to be 2.27 (), 0.94 (), and 2.61% (), respectively.
Table 1. Preliminary phytochemical studies on various extract of P. rosea.
Acute toxicity studies
No mortality and changes in the behavior was observed in the treatment groups up to 2000 mg/kg body weight and from the results 400 mg/kg dose was chosen for further experimentation as the maximum dose.
Effect of extract on the estrous cycle of rats
The present study indicated that the acetone extract of P. rosea stems are responsible for the antifertility effect. Treatment of rats with acetone extract for 5 days prolonged the estrous cycle significantly, as indicated in . The estrous cycle in rats treated with acetone extract showed reduced duration of estrous and metestrous phases and, on the other hand, it was also characterized by a prolongation of the diestrous phase. The estrous cycle was found to be reversible on withdrawal of the treatment (). All remaining extracts were found inactive when compared to control ().
Figure 5. Vaginal smear of rats on 4-day estrous cycle. CXR III camera (x 400), Control: 1st day (A), 2nd day (B), 3rd day (C), 4th day (D).
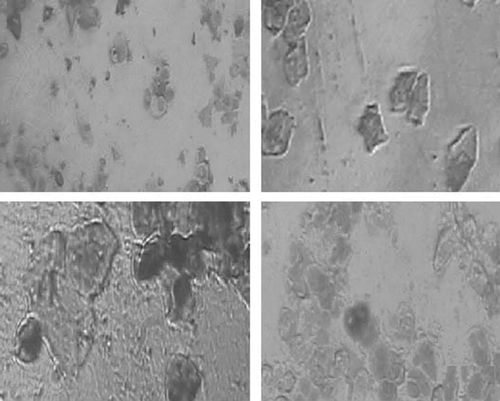
Table 2. Effect of treatment of various extracts of stem on estrous cycle for 5 days in rats.
Table 3. Effect of post-treatment of various extracts of stem on estrous cycle for 15 days in rats.
Estrogenic and antiestrogenic activity
The effects of acetone extract of P. rosea stem in immature rats uteri are shown in . Oral administration of the extract at 200 and 400 mg/kg body weight caused a significant increase in the uterine weight of immature rats. When compared to the control rats (), the height of the endometrial epithelium and thickness of the endometrium was significantly increased (). The epithelium of the endometrium consisted of spindle shaped cells with basal nuclei and the endometrial glands were dilated. The stroma consisted of loose and edematous fibroblast type cells with edema (). The control rats showed closed vagina, at the same time the treated rats showed an open vagina.
Figure 6. Photomicrograph of the transverse section of uterus of control rat showing the endometrium.
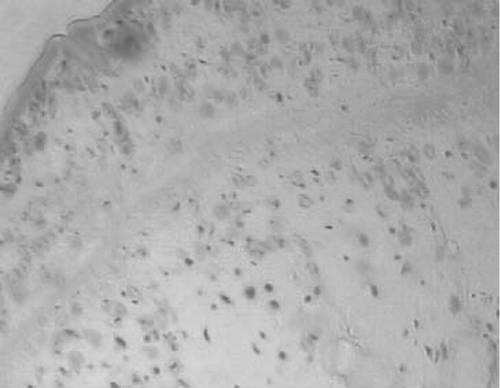
Figure 7. Photomicrograph of the transverse section of uterus of ethinyl estradiol (0.02 mg/kg p.o.) treated rat, showing proliferation stage.
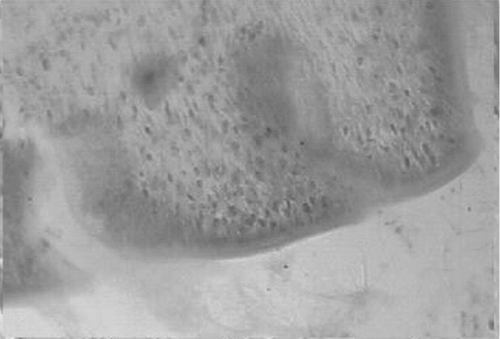
Figure 8. Photomicrograph of the transverse section of uterus of acetone extract (400 mg/kg p.o) treated rat, showing the sloughing of the endometrial lining of epithelium.
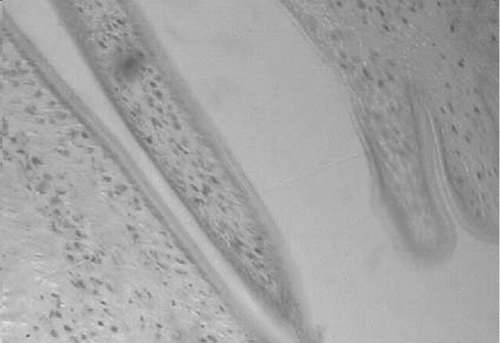
Table 4. Estrogenic and antiestrogenic activity of acetone extract of P. rosea stem.
The administration of acetone extract aggravated a significant increase in the uterine wet weight, signifying estrogenic activity, but when given together with ethinyl estradiol, the estrogenic activity produced by ethinyl estradiol alone was reduced ().
Discussion
The acetone extract of stems of P. rosea exhibited significant antifertility activity. The duration of estrous cycle in rats is normally 4–5 days. When observed using a microscope, the vaginal smear during a routine rat estrous cycle shows three cell types () and, depending on the presence and absence of these cell types and their relative proportion, the stages of estrous cycle of rats can be determined. Of the five extracts tested for antiovulatory activity, only the acetone extract produced a temporary and reversible modification of the estrous cycle. The prolongation in the diestrous phase explains the remote possibility of rats getting pregnant. The reversible nature of the antifertility activity of the extract is explained through the observation that there was no significant change in the diestrous and estrous cycle after withdrawing the extract from those of the treated animals. As a result, the extract provoked inhibition of ovulation with consequent reduction of the cyclicity. The estrous cycle and the shift in different stages are mainly governed by the synthesis of ovarian estrogen which, in turn, is controlled by the secretion of pituitary gonadotropins and hypothalamic-releasing factor (CitationCircosta et al., 2001).
As the acetone extract showed antiovulatory activity, it was further studied for estrogenic and antiestrogenic activity. The extract also exhibited estrogenic activity as shown by the significant increase in diameter of the uterus, uterine weight, and thickness of the endometrial epithelium, when compared to the control. It was also observed that the acetone extract suppressed the action of ethynyl estradiol when administered together. The extract showed a significant estrogen-like activity when given alone, but with ethinyl estradiol, it exhibited a slight antiestrogenic nature. This indicates that extract acted as a competitative antagonist to the much more potent ethinyl estradiol (CitationBadami et al., 2003).
Preliminary phytochemical studies indicated the presence of tannins, flavonoids, triterpenoids, and napthaquinone in the acetone extract. According to the literature, flavonoids and plumbagin (napthaquinone) are known to exhibit antifertility activity (CitationVaidya et al., 2006; CitationBadami et al., 2003; CitationDevarshi et al., 1991). In our study also, the activity may be due to the presence of flavonoids and napthaquinones. The petroleum ether and chloroform extract too showed the presence of napthaquinones, but the activity was not found in these extracts. The possible reason may be that a sufficient concentration or the particular active constituent (flavonoid or napthaquinone) responsible for antifertility activity is not present in petroleum ether, chloroform, ethanol, and aqueous extract, or the activity of the acetone extract may be due to synergism produced by napthaquinone along with a flavonoid.
Conclusion
The results of the present study indicate that the acetone extract of P. rosea stem have significant antifertility activity. The acetone extract of stems of this plant could be used to induce abortion and can further be developed into a contraceptive. The stems could serve as an alternative medicine to the use of its roots as abortifacient and uprooting of this plant could be avoided.
Acknowledgement
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Anonymous (1985): The Wealth of India. New Delhi, National Institute of Science Communications and Information Resources (NISCAIR), Council of Scientific and Industrial Research (CSIR) p. 162.
- S Badami, R Aneesh, S Sankar, MN Satishkumar, B Suresh, and S Rajan. (2003). Antifertility activity of Derris brevipes variety coriacea. J Ethnopharmacol 84:99–104.
- C Circosta, R Sanogo, and F Occhiuto. (2001). Effects of Calotropis procera on estrous cycle and on estrogenic functionality in rats. Il Farmaco 56:373–378.
- P Devarshi, S Patil, and A Kanase. (1991). Effect of Plumbago rosea root powder induced pre implantationary loss and abortion on uterine luminal proteins in albino rats. Indian J Exp Biol 29:521–522.
- PU Devi, FE Solomon, and AC Sharada. (1994). In vivo tumor inhibitory and radiosensitizing effects of an Indian medicinal plant, Plumbago rosea on experimental mouse tumors. Indian J Exp Biol 32:523–528.
- NK Dhal, NC Rout, and M Thirunavoukkarasu. (2000). Plumbago indica Linn. (Plumbaginaceae): A specific case study for birth control among the Jani tribe of Orissa. Ethnobotany 12:27–28.
- AIC Dorni, KS Vidyalakshmi, RV Hannah, GV Rajamanickam, and GP Dubey. (2006). Antiinflammatory activity of Plumbago capensis. Phcog Mag 2:239–243.
- SP Hiremath, K Rudresh, S Badami, SB Patil, and SR Patil. (1999). Post-coital antifertility activity of Acalypha indica L. J Ethnopharmacol 67:253–258.
- JV Kamath, and AC Rana. (2002). Preliminary study on antifertility activity of Calotropis procera roots in female rats. Fitoterapia 73:111–115.
- KR Khandelwal (2005): Practical Pharmacognosy. Pune, Nirali Prakashan, pp. 149–157.
- RK Kritikar, DB Basu (1975).Indian Medicinal Plants. DelhiJayyed Press, pp. 1465–1470.
- S Manimaran, SP Dhanabal, S Sudhakarraja, and B Suresh. (2007). Estimation of Harmaline content in Passiflora edulis by HPTLC technique. Indian J Pharm Edu Res 41:159–160.
- NK Mary, BH Babu, and J Padikkala. (2003). Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomedicine 10:474–482.
- MK Misra, SK Behera, A Panda, and SK Behera. (2006). Medicinal plants used by the Kandhas of Kandhamal district of Orissa. Ind J Trad Knowledge 5:519–528.
- SC Nath, and J Purkayastha. (2004). Biological activity of ethnomedical claim of some plant species of Assam. Ind J Trad Knowledge 5:229–236.
- V Ravikanth, and VD Prakash. (1999). Phytochemical and pharmacological aspects of Plumbago rosea. Indian Drugs 36:724–730.
- HN Sarma, and HC Mahanta. (2000). Effects of composite root extract on rat granulosa cells: A transmission electron microscopic observations. J Exp Zool 3:217–221.
- P Vaidya, S Padmashali, HM Vagdevi, and ND Sathyanarayana. (2006). Antifertility effect of the plant Balanites roxburghii (Balanitaceae) in female rats. Ind J Pharm Sci 3:347–351.