Abstract
This study evaluated the in vitro antioxidant potential of methanol extract of Lippia nodiflora Mich. (Verbenaceae) (MELN). The different antioxidants assays, including total antioxidant activity, reducing power, free radical, superoxide anion radical, hydroxyl radical, hydrogen peroxide, nitric oxide scavenging, and total phenolic content, were studied. MELN exhibited potent total antioxidant activity that increased with increasing amount of extract concentration (50, 100, 200, and 400 μg/mL), which were compared with standard drug α -tocopherol (400 μg/mL). The different concentrations of MELN and α -tocopherol showed inhibition of 49.07%, 58.96%, 63.07%, 68.29%, and 74.59%, respectively, on peroxidation in linoleic acid emulsion. In addition, MELN had effective reducing power, free radical scavenging, superoxide anion radical scavenging, hydroxyl radical scavenging, hydrogen peroxide radical scavenging, and nitric oxide scavenging activity, and total phenolic content depending on concentration. These various antioxidant activities were compared with standard antioxidants such as BHA, BHT, catechin, and α -tocopherol.
Introduction
Antioxidants provide protection for living organisms from damage caused by uncontrolled production of reactive oxygen species (ROS) and the concomitant lipid peroxidation, protein damage, and DNA strand breaking (CitationGhosal et al., 1996). Current interest is focused on the potential role of antioxidants and antioxidant enzymes in the treatment and prevention of atherosclerosis, heart failure, neurodegenerative disorders, aging, cancer, diabetes mellitus, and several other diseases (CitationAjitha & Rajnarayana, 2001).
Antioxidants are added to a variety of foods to prevent or deter free radical-induced lipid peroxidation, which is responsible for the development of off-flavors and the undesirable chemical compounds in food (CitationAngelo, 1996). The free radicals can also be generated in biological systems in the form ROS (CitationHalliwell et al., 1995). These ROS cause destructive and irreversible damage to the components of a cell, such as lipids, proteins, and DNA (CitationLopaczyski & Zeisel, 2001). Although normal cells possess antioxidant defense systems against ROS, the continuous accumulation of ROS in the cells induces diseases such as cancer and aging (CitationMates & Sanchez-Jimenez, 2000).
ROS are formed and degraded by all aerobic organisms. ROS can readily react with most biomolecules including protein, lipids, lipoproteins, and DNA. Exogenous chemical and endogenous metabolic processes in the human body or in the food system might produce highly reactive oxygen species, which are capable of oxidizing biomolecules, resulting in tissue damage and cell death (CitationNordberg & Arner, 2001). When the mechanism of antioxidant protection becomes unbalanced by exogenous and endogenous factors, it results in inflammation, diabetes, genotoxicity, cancer, and accelerating aging (CitationBuyukokuroglu et al., 2001).
Antioxidant supplements or foods containing antioxidants may be used to help the human body reduce oxidative damage. The most commonly used antioxidants are BHA, BHT, propyl gallate, and tert-butyl-hydroquinone (CitationGulcin et al., 2004). However, they have been suspected of being responsible for liver damage and carcinogenesis in laboratory animals. Therefore, the development and use of more effective antioxidants is desired.
Traditional medicine worldwide is being reevaluated by extensive research on different plant species and their therapeutic principles. Plants produce antioxidants to control the oxidative stress caused by sunbeams and oxygen; they can represent a source of new compounds with antioxidant activity.
Lippia nodiflora Mich. (Verbenaceae) is a creeping, many-branched herb with small white flowers; a weed of wet ground and grassy pastures (CitationGamble, 1957; CitationChopra et al., 1958). The herb is known as “poduthalai” in Tamil, “kattuttippali” in Malayalam, and “bhuiokra” in Hindi. The plant is distributed throughout India, Ceylon, Baluchistan, and Africa. The plant is used as a diuretic and aphrodisiac and as a treatment for heart disease, ulcers, bronchitis, fevers, and colds (CitationKirthikar & Basu, 1975). The plant is used for boils, for treatment of indigestion in children, and in women after delivery (CitationNadkarni, 1954; CitationChopra et al., 1956). The herb possesses a cooling characteristic, is diuretic, and is used as a cure for knee joint pain (CitationAnonymous, 1962, Citation1986).
This study evaluated the total antioxidant activity, reducing ability, free radical scavenging, superoxide anion radical scavenging, nitric oxide radical scavenging, hydroxyl radical scavenging, hydrogen peroxide radical scavenging activity, and total phenol content of methanol extract of Lippia nodiflora. An important objective of this research was to compare in vitro antioxidative potentials of methanol extract of Lippia nodiflora (MELN) with those of standard antioxidants such as α -tocopherol, BHA, and BHT.
Materials and Methods
Plant material
The whole plant of Lippia nodiflora was collected during January 2005 from wet areas of Mallasamudram, Namakkal district, Tamilnadu, India. The plant material was taxonomically identified by Prof. Revenna, H.O.D, Department of Botany, Kuvempu First Grade College, Channapatna, Karnataka, India. A voucher specimen (no. DAK/JU04/2005) is maintained in our laboratory for future reference.
Preparation of plant extract
The plant material was shade-dried with occasional shifting and then powdered with a mechanical grinder, passing through sieve #40, and stored in a tight container. The dried powder material (350 g) was defatted with petroleum ether (60–80°C), and the defatted powder material thus obtained was further extracted with methanol for 72 h in a Soxhlet apparatus. The solvent was distilled under reduced pressure, and the resulting semisolid mass was vacuum dried using rotary flash evaporator to yield a solid residue (21.42% w/w). The preliminary phytochemical analysis was performed to identify the phytoconstituents present in the extract (CitationKokate, 1994).
Chemicals
Ammonium thiocyante was purchased from E. Merck (Mumbai, India) and Stigma Chemical Co. Ltd., (Mumbai, India). Ferrous chloride, ferric chloride, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), nicotinamide adenine dinucleotide (NADH), EDTA, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), α -tocopherol, curcumin, catechin, nitroblue tetrazolium (NBT), thiobarbituric acid (TBA), trichloroacetic acid (TCA), phenazine methosulfate (PMS), and potassium ferric cyanide were purchased from Sigma Chemical Co. Ltd, (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade.
Antioxidant activity determination in linoleic acid emulsion using the ferric thiocyanate method
The antioxidant activity was determined according to the ferric thiocyanate method in linoleic acid emulsion (CitationMitsuda et al., 1996). MELN (10 mg) was dissolved in 10 mL of water. MELN at different concentrations (50, 100, 200, and 400 μg/mL) or standard sample (400 μg/mL) in 2.5 mL of potassium phosphate buffer (0.04 M, pH 7.0) was added to a linoleic acid emulsion (2.5 mL). The linoleic acid emulsion (50 mL) consisted of 175 μ g Tween 20, 155 μ l linoleic acid, and 0.04 M potassium phosphate buffer (pH 7.0). The control (50 mL) consisted of 25 mL linoleic acid emulsion and 25 mL potassium phosphate buffer (0.04 M, pH 7.0). The mixed solution was incubated at 37°C in a glass flask protected from light. After the mixture was stirred for 3 min, the peroxide value was determined by reading the absorbance at 500 nm in a spectrophotometer (Genesys 10UV; Thermo Electron Corporation, Minnesota, USA), after reacting with FeCl2 and thiocyanate at intervals during the incubation. During the linoleic acid oxidation, peroxides formed, which oxidized Fe2 + to Fe3 +. The Fe3 + ions form a complex with SCN−, and this complex has maximum absorbance at 500 nm. Therefore, high absorbance indicates high linoleic acid oxidation. The solutions, without added extracts, were used as blank samples. All antioxidant activity data were the average of triplicate analyses. The inhibition of lipid peroxidation was calculated by following equation:
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the sample of MELN extract
Total reduction capability by Fe3+-Fe2+ transformation
The total reducing power of the MELN was determined according to the method of CitationOyaizu (1986). Briefly, different concentrations of MELN (20, 40, 60, 80, and 100 μg/mL) in 1 mL of distilled water were mixed with phosphate buffer (2.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%). The mixture was incubated at 50°C for 20 min. Trichloroacetic acid (2.5 mL, 10%) was added to the mixture, which was then centrifuged for 10 min at 1000 × g (Remi T8A, Mumbai, India). The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm using a UV-Vis spectrophotometer (Genesys 10UV; Thermo Electron Corporation). Higher absorbance of the reaction mixture indicated greater reducing power.
Free radical scavenging activity measured by 1,1-diphenyl-2-picryl-hydrazyl
The free radical scavenging activity of MELN extracts was measured by 1,1-diphenyl-2-picryl-hydrazyl (DPPH•) using the method of CitationBlois (1958). Briefly, an 0.1 mM solution of DPPH• in methanol was prepared, and 1 mL of this solution was added to 3 mL of MELN extract solution in water at different concentrations (2, 4, 6, 8, 10, and 15 μg/mL). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then, the absorbance was measured at 517 nm using a UV-Vis spectrophotometer (Genesys 10UV; Thermo Electron Corporation). Lower absorbance values of the reaction mixture indicated higher free radical scavenging activity. The capability to scavenge the DPPH radical was calculated using the following equation:
where A0 is the absorbance of the control reaction, and A1 is the absorbance in the presence of the sample of MELN.
Superoxide anion radical scavenging activity in PMS-NADH system
Measurement of superoxide anion scavenging activity of MELN extracts was based on the method described by Nishimiki and colleagues (1972) with slight modification. One milliliter of nitroblue tetrazolium (NBT) solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4), 1 mL of nicotinamide adenine dinucleotide (NADH) solution (468 μM in 100 mM in 100 mM phosphate buffer, pH 7.4), and various concentrations (20, 40, 60, 80, and 100 μg/mL) of sample solution of MELN in water were mixed. The reaction started by adding 100 μL phenazine methosulfate (PMS) solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4) to the mixture. The mixture was incubated at 25°C for 5 min, and the absorbance at 560 nm was measured against blank samples. A decreased in the absorbance of reaction sample indicated increased superoxide anion scavenging activity. BHA and BHT were used as standard drugs. The percent inhibition of superoxide anion generation was calculated using the following formula:
where A0 is the absorbance of the control, and A1 is the absorbance of MELN extracts and standards.
Nitric oxide radical scavenging assay
In vitro nitric oxide generated from sodium nitroprusside in aqueous solution at physiologic pH interacts with oxygen to produce nitrite ions, which was measured by Griess reaction (CitationMarcocci, 1994). Reaction mixture (3 mL) containing sodium nitroprusside (10 mM) in phosphate-buffered saline (PBS) and various concentrations (20, 40, 60, 80, and 100 μg/mL) of the plant extract were incubated at 25°C for 150 min. At the end of the incubation, 0.5 mL of the reaction mixture was removed and 0.5 mL of Griess reagent (1% sulfanilamide, 2% H3PO4, and 0.1% naphthylethylene diamine dihydrochloride) was added. The absorbance of chromophore formed was measured at 546 nm. The percentage inhibition of nitrite oxide generated was measured by comparing the absorbance values of control and test compounds.
Hydroxyl radical (HO•) scavenging assay
The ability of different concentrations of Lippia nodiflora to scavenge the hydroxyl radical generated by the Fenton reaction was measured according to the method of CitationElizabeth and Rao (1990). The hydroxyl radical attacks deoxyribose, which eventually results in thiobarbituric acid reacting substance (TBARS) formation. The reaction mixture contained in a final volume of 1.0 mL [100 μ L of 2-deoxy6-2-ribose (28 mM in KH2PO4-KOH buffer, 20 mM, pH 7.4), different concentrations of MELN (20, 40, 60, 80, and 100 μg/mL) and the reference compound in KH2PO4-KOH buffer (20 mM, pH 7.4), 200 μ L of 1.04 mM EDTA and 200 μM of FeCl3 (1:1 v/v), 100 μ L of 1.0 mM of H2O2 and 100 μ L of 1.0 mM ascorbic acid] was incubated at 37°C for 1 h. Thiobarbituric acid (1%) (1 mL) and 1.0 mL of trichloroacetic acid (2.8%) were added to the test tubes and incubated at 100°C for 20 min. After cooling, absorbance was measured at 532 nm against a control sample containing deoxyribose and buffer. Deoxyribose degradation was measured as TBARS by the method of CitationOhkawa et al. (1979), and percentage inhibition was calculated and concentration needed for 50% inhibition was determined by comparing the results of the test compounds and control. Reactions were carried out in triplicate. Catechin was used as a reference control.
Hydrogen peroxide radical scavenging assay
The capacity of MELN to inhibit hydrogen peroxide was determined (CitationJayaprakasha & Jaganmohan Rao, 2004). A solution of hydrogen peroxide (20 mM) was prepared in phosphate buffer solution (PBS, pH 7.4). Various concentrations (25, 50, and 75 μg/mL) of 1 mL of the extracts or standard in methanol were added to 2 mL of H2O2 solutions in PBS. The absorbance of hydrogen peroxide was measured at 230 nm after 10 min against a blank solution that contained phosphate buffer without hydrogen peroxide. The percentage of H2O2 scavenging of MELN and standard compound α -tocopherol was calculated:
where A0 is absorbance of the control, and A1 is absorbance of the presence of MELN extracts or standards.
Determination of total phenolic compounds
The total phenolic compounds in the MELN at different concentrations were determined (CitationSlinkard & Singleton, 1977) with Folin-Ciocalteu reagent using pyrocatechol as a standard phenolic compound. Briefly, 1 mg of extract solution (1000 μ g of extract) was placed in a 100-mL Erlenmeyer flask diluted with distilled water (46 mL). Folin-Ciocalteu reagent (1 mL) was added, and the contents of the flask were mixed thoroughly. After 3 min, 3 mL of Na2CO3 (2%) was added, then the mixture was allowed to stand for 2 h with intermittent shaking. The absorbance was measured at 760 nm in a spectrophotometer (Genesys 10UV; Thermo Electron Corporation). The amount of total phenolic compounds in the MELN was determined in micrograms of pyrocatechol equivalent, using the equation obtained from the standard pyrocatechol graph:
Statistical analysis
Experimental results were mean ± SEM of three parallel measurements. Statistical analysis was estimated using Student's t-test followed by ANOVA method. The values for p < 0.05 were considered as significant and values for p < 0.001 as very significant.
Results and Discussion
The qualitative chemical analysis of MELN showed the positive result for the presence of glycosides, flavonoids, triterpenoids, tannins, and steroids. Antioxidant methods and modifications have been proposed to evaluate antioxidant characteristics and to explain how antioxidants function. Of these, antioxidant activity, reducing power, free radical scavenging, superoxide anion radical scavenging, nitric oxide radical inhibition, hydroxyl radical scavenging, and hydrogen peroxide scavenging activities and estimation of phenol content are most commonly used for the evaluation of the total antioxidant behavior of extracts.
Effect on total antioxidant activity
The antioxidant activity of linoleic acid peroxidation due to the presence of phytoconstituents such as flavonoids and biflavones has been reported (CitationWang & Wixon, 1999). Therefore, the current study suggests the antioxidant activity of MELN may be attributed to the reduction of hydroperoxides, inactivation of free radicals, chelation of metal ions or combination thereof, or presence of phytoconstituents.
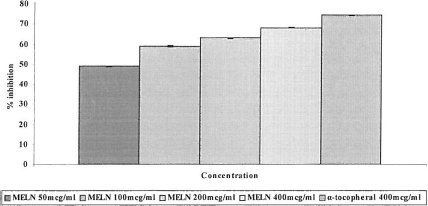
The antioxidant activity of MELN extracts was determined by the thiocyanate method. The various concentrations of MELN (50, 100, 200, and 400 μg/mL) exhibited effective antioxidant activity on peroxidation in linoleic acid emulsion and is shown in . The antioxidant activity of MELN increased with increasing concentration of sample. The inhibition of peroxidation in the linoleic acid system of MELN and α-tocopherol were 49.07%, 58.96%, 63.07%, 68.29%, and 74.59%, respectively. MELN at a concentration of 400 μg/mL showed activity nearly equal to that of a 400 μg/mL concentration of α -tocopherol. The results indicate that the methanol extract of Lippia nodiflora significantly (p < 0.05) inhibits linoleic acid peroxidation compared with control. This method was used to estimate the peroxide level during the initial stages of lipid oxidation.
Effect on reductive ability
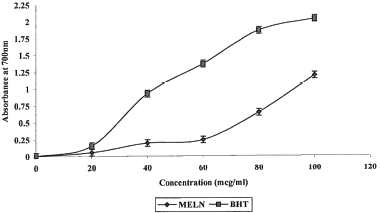
For the measurement of the reductive ability, we investigated the Fe3+-Fe2+ transformation in the presence of extract. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity (CitationMeir et al., 1995). The antioxidant activity of putative antioxidants has been attributed to various mechanisms, among which are the prevention of chain initiation, the binding of transition metal ion catalysts, decomposition of peroxides, the prevention of continued hydrogen abstraction, the reductive capacity, and radical scavenging (CitationDiplock, 1997). Like the antioxidant activity, the reducing power of MELN increased with increasing concentration of sample. shows the reductive capabilities of the MELN compared with BHT. All MELN extract concentrations tested showed higher activities than the control, and these differences were statistically significant (p < 0.001).
Effect on scavenging of DPPH radical
The stable DPPH radical model is a widely used, relatively quick method for the evaluation of free radical scavenging activity. The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen-donating ability. DPPH• is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule (CitationSoares et al., 1997). The absorption maximum of a stable DPPH radical in methanol was at 517 nm. The decrease in absorbance of DPPH radical caused by antioxidants, because of the reaction between antioxidant molecules and radical progresses, results in the scavenging of the radical by hydrogen donation. It is visually noticeable as a change in color from purple to yellow. Hence, DPPH• is usually used as a substrate to evaluate the antioxidative activity of antioxidants (CitationChang et al., 2002). It has been reported that oxidative stress, which occurs when free radical formation exceeds the body's ability to protect itself, forms the biological basis of chronic conditions such as arteriosclerosis (CitationFatimah et al., 1998).
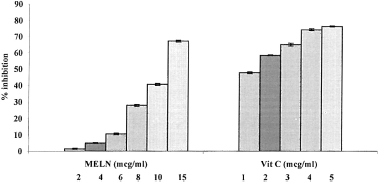
Based on the data obtained from this study, MELN is an effective free radical inhibitor or scavenger, as well as a primary antioxidant that reacts with free radicals, which may limit free radical damage occurring in the human body. illustrates a significant (p < 0.001) decrease in the concentration of DPPH radicals due to the scavenging ability of the extract and standard. Vitamin C was used as standard. Free radical scavenging activity also increased with increasing concentration in the range 2–15 μg/mL. IC50 values of MELN and ascorbic acid were 12.03 and 0.98 μg/mL, respectively.
Effect on superoxide anion radical scavenging activity
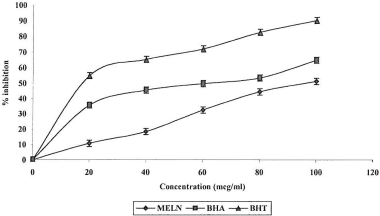
Superoxide anion radicals (O·−2) are formed by activated phagocytes such as monocytes, macrophages, eosinophil's and neutrophills, and the production of O·−2 is an important factor in the killing of bacteria by phagocytes. In the PMS-NADH-NBT system, superoxide anion, derived from dissolved oxygen from the coupling reaction of PMS-NADH, reduces NBT (CitationGulcin et al., 2002). The decrease in absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. shows the percent inhibition of superoxide radical generation by 20, 40, 60, 80, and 100 μg/mL of MELN compared with the same doses of BHA and BHT. All the concentrations of MELN have a significant level (p < 0.001) of superoxide radical scavenging activity compared with control. The IC50 values of MELN, BHA, and BHT were found to be 94.28, 60.11, and 7.67 μg/mL, respectively.
Effect on nitric oxide (NO·) scavenging activity
Nitric oxide and superoxide anion cause ischemic renal injury separately, and these radicals work together to bring about further damage. The toxicity and damage caused by NO• and O2•− is multiplied as they react to produce reactive peroxynitrite (ONOO−), which leads to serious toxic reactions with biomolecules such as protein, lipids, and nucleic acids (CitationYermilov et al., 1995). High concentration of nitric oxide (NO) has deleterious effects, so it is necessary that the production of NO be tightly regulated (CitationBeasley et al., 1991). When NO is produced by macrophages, the nitric oxide radical can be converted into peroxynitrites, which will cause diverse chemical reactions in a biological system including nitration of tyrosine residue of protein, triggering lipid peroxidation, inactivation of aconites, inhibition of mitochondrial electron transport, and oxidation of biological thiol compound (CitationMaeda & Akaike, 1998).
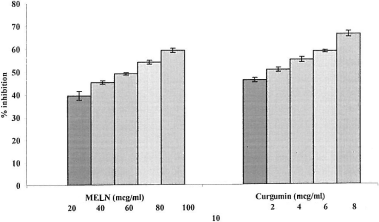
Suppression of NO• released may be partially attributed to direct NO• scavenging, as all concentrations of MELN decreased the amount of nitrite generated from the decomposition of sodium nitroprusside in vitro. A nitric oxide radical generated from sodium nitroprusside at physiologic pH was found to be inhibited by MELN. illustrates the percentage inhibition of nitric oxide production. Curcumin was used as a reference compound. The concentration of MELN necessary for 50% inhibition was found to be 62.26 μg/mL. However, 3.75 μg/mL was required for curcumin. The extract showed significant level (p < 0.001) of inhibition of nitric oxide production by activated peritoneal macrophages with in vitro conditions.
Effect on hydroxyl radical scavenging activity
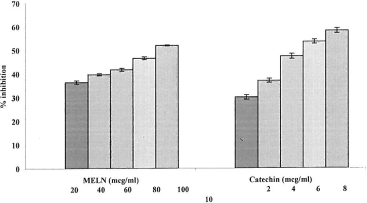
The ability to scavenge the •OH radical was determined using the ascorbic–acid iron–EDTA model OH-generating system. This is a totally aqueous system in which ascorbic acid, iron, and EDTA interact to generate the hydroxyl radical (CitationKlein et al., 1981). Hydroxyl radical scavenging capacity of an extract is directly related to its antioxidant activity and it was markedly decreased by the MELN. The results are illustrated in . This indicated the significant (p < 0.001) hydroxyl radical scavenging activity of the extract. The IC50 of MELN was found to be 95.22 μg/mL. Catechin was used as standard and the IC50 was found to be 7.30 μg/mL.
Effect on hydrogen peroxide (H2O2) scavenging activity
H2O2 is a weak oxidizing agent and can inactivate a few enzymes directly, usually by oxidation of essential thiol (–SH) groups. Hydrogen peroxide can cross cell membranes rapidly; once inside the cell, H2O2 can probably react with Fe2− and possibly Cu2− ions to form hydroxyl radical, and this may be the origin of many of its toxic effects (CitationMiller et al., 1993). Hydrogen peroxide converts into the singlet oxygen (1O2) and hydroxyl radicals, which then become very powerful oxidizing agents. Not only 1O2 and HO• but also H2O2 can cross membranes and may oxidize a number of compounds. Although hydrogen peroxide itself is not very reactive, it can sometimes cause cytotoxicity by giving rise to hydroxyl radicals in the cell. Thus, removing H2O2 is very important throughout food systems (CitationHalliwell, 1991).
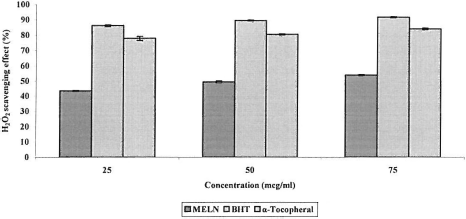
It is therefore biologically advantageous for cells to control the amount of hydrogen peroxide that is allowed to accumulate. The differences in H2O2 scavenging capacities at various concentrations of MELN may be attributed to the structural features of their active components, which determine their electron donating abilities. displays the ability of MELN to effectively scavenge hydrogen peroxide, where it is compared with that of BHT and α-tocopherol as standards. MELN at various concentrations (25, 50, and 75 μg/mL) exhibited 43.55%, 49.43%, and 53.91% hydrogen peroxide scavenging activity, respectively, while, at the same concentration, BHT and α -tocopherol showed 86.25%, 89.64%, 91.95% and 77.97%, 80.60%, 84.26% activity, respectively. The correlation between MELN values and those of the controls was statistically significant (p < 0.05). MELN was capable of scavenging hydrogen peroxide in a concentration-dependent manner.
Effect of total phenolic compounds
Phenolic constituents are very important in plants because of their scavenging ability due to their hydroxyl groups (CitationHatano et al., 1989). A number of studies have focused on the biological activities of phenolic compounds, which are potential antioxidants and free radical scavengers (CitationRice-Evans et al., 1995). In addition, it has been reported that phenolic compounds are associated with antioxidant activity and play an important role in stabilizing lipid peroxidation (CitationYen et al., 1993). One milligram of each concentration of MELN contained 95.03 μ g of pyrocatechol equivalents of phenols. These phenolic compounds may contribute directly to the antioxidative action. It has been suggested that up to 1 g of polyphenolic compounds (from a diet rich in fruits and vegetables) ingested daily has inhibitory effects on mutagenesis and carcinogenesis in humans (CitationTanaka et al., 1998).
Conclusions
Based on the results of this study, it is clear that MELN has powerful in vitro antioxidant capacity against various antioxidant systems. From the results, it can be concluded that the antioxidant activity of MELN was concentration dependent with inhibition of lipid peroxidation. From the above assays, the possible mechanism of antioxidant activity of MELN includes reducing ability, hydrogen-donating ability, and scavenging of superoxide, nitric oxide, hydroxyl, hydrogen peroxide, and free radicals, which may be due to the presence of phytoconstituents such as flavonoids and polyphenols present in the methanol extract of Lippia nodiflora.
Acknowledgement
D.A. is grateful to AICTE, New Delhi, India, for providing financial support to this work through the Quality Improvement Programme.
References
- M Ajitha, and K Rajnarayana. (2001). Role of oxygen free radicals in human diseases. Indian Drugs 38:545–554.
- A J Angelo. (1996). Lipid peroxidation in food. Crit Rev Food Sci Nutr 36:175–224.
- Anonymous (1962): The Wealth of India-Raw Materials, Vol. VI. New Delhi, Publication and Information Directorate, Council of Scientific and Industrial Research, pp. 142–143.
- Anonymous (1986): The Useful Plants of India, Third edition. New Delhi, Publication & Information Directorate, Council of Scientific & Industrial Research, New Delhi, p. 450.
- D Beasley, J H Schwartz, and B M Brenner. (1991). Interleukin induces prolonged l-arginine dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest 87:602–608.
- M S Blois. (1958). Antioxidant determinations by the use of a stable free radical. Nature 26:1199–1200.
- M E Buyukokuroglu, I Gulcin, M Oktay, and O I Kufrevioglu. (2001). In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 44:491–495.
- L W Chang, W J Yen, S C Huang, and P D Duh. (2002). Antioxidant activity of sesame coat. Food Chem 78:347–354.
- R N Chopra, I C Chopra, K L Handa, and L D Kapur. (1958): Indigenous Drugs of India, Second edition. Calcutta, U. N. Dhur & Sons Private Limited, p. 513.
- R N Chopra, S L Nayar, and I C Chopra. (1956): Glossary of Indian Medicinal Plants. New Delhi, C. S. I.R, p. 155.
- A T Diplock. (1997). Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease?. Free Radical Res 27:511–532.
- K Elizabeth, and M NA Rao. (1990). Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–240.
- Z I Fatimah, Z Zaiton, M Jamaludin, M T Gapor, M I Nafeeza, and O Khairul. (1998): Effect of estrogen and palm vitamin E on malondialdehyde levels toward the development of arteriosclerosis in the New Zealand white rabbit. In: L Packer, and S H Ong. eds. Biological Oxidants and Antioxidants: Molecular Mechanism and Health Effects. Champaign, IL, AOCS Press, p. 22.
- J S Gamble. (1957): Flora of the Presidency of Madras, Vol. II. Calcutta, Botanical Survey of India, p. 762.
- S Ghosal, V K Tripati, and S Chauhan. (1996). Active constituents of Emblica officinalis, Part I, the Chemistry and antioxidative effects of two new hydrolysable tannins, emblicanin A and B. Indian J Chem (B) 35:941–948.
- I Gulcin, M Oktay, O I Ku Freviolglu, and A Aslan. (2002). Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 79:325–329.
- I Gulcin, S Beydemir, A H Ahmet, E Mahfuz, and B M Emin. (2004). In vitro antioxidant properties of morphine. Pharmacol Res 49:59–66.
- B Halliwell. (1991). Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med 91:4S–22S.
- B Halliwell, R Aeschbach, J Loliger, and O I Aruoma. (1995). The charecterization of antioxidants. Food Chem Toxicol 33:601–617.
- T Hatano, R Edamatsu, A Mori, Y Fujita, and E Yasuhara. (1989). Effect of interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chem Pharm Bull 37:2016–2021.
- G D K Jayaprakasha, and L Jaganmohan Rao. (2004). Antioxidant activities of flavidin in different in vitro model system. Bioorg Med Chem 12:5141–5146.
- K R Kirthikar, and B D Basu. (1975): Indian Medicinal Plants, Vol. III. Dehradun, Bishen Mahendra Pal Singh, pp. 1915–1917.
- S M Klein, G Cohen, and A I Cederbaum. (1981). Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical generating systems. Biochemistry 20:6006–6012.
- C K Kokate. (1994): Practical Pharmacognosy, Fourth edition. Delhi, Vallabh Prakashan, pp. 107–112.
- W Lopaczyski, and S H Zeisel. (2001). Antioxidants, programmed cell death, and cancer. Nutr Res 21:295–307.
- H Maeda, and T Akaike. (1998). Nitric oxide and oxygen radicals in infection, inflammation, and cancer. Biochemistry (USSR) 63:2408–2416.
- L Marcocci, J J Maguire, M T Droy-Lefaix, and L Packer. (1994). The nitric oxide scavenging activity of Ginko biloba extract. EGB 761. Biochem Biophys Res Commun 201:748–755.
- J M Mates, and F M Sanchez-Jimenez. (2000). Role of reactive oxygen species in apoptosis: Implications for cancer therapy. Int. J. Biochem. Cell Biol 32:57–170.
- S Meir, J Kanner, B Akiri, and S P Hadas. (1995). Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J Agric Food Chem 43:1813–1819.
- M J Miller, H Sadowska-krowicka, S Chotinaruemol, J L Kakkis, and D A Clark. (1993). Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther 264:11–16.
- H Mitsuda, K Yuasumoto, and K Iwami. (1996). Antioxidation action of indole compounds during the autoxidation of linoleic acid. Eiyo to Shokuryo 19:210–214.
- A K Nadkarni. (1954): Indian Materia Medica. Bombay, India, Popular Prakashan, p. 746.
- M Nishimiki, N A Rao, and K Yagi. (1972). The occurance of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophy Res Commun 46:849–853.
- J Nordberg, and E SJ Amer. (2001). Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312.
- H Ohkawa, N Ohishi, and K Yagi. (1979). Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358.
- M Oyaizu. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315.
- C Rice-Evans, N J Miller, G P Bolewell, P M Bramley, and J B Pridham. (1995). The reactive antioxidants activities of plant derived poly phenolic flavonoids. Free Radic Res 22:375–383.
- K Slinkard, and V L Singleton. (1977). Total phenol analyses: Automation and comparison with manual methods. Am J Enol Vitic 28:49–55.
- J R Soares, T CP Dins, A P Cunha, and L M Almeida. (1997). Antioxidant activity of some extracts of. Thymus zygis. Free Radic Res 26:469–478.
- M Tanaka, C W Kuei, Y Nagashima, and T Taguchi. (1998). Application of antioxidative mailrad reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishi 54:1409–1414.
- C Wang, and R Wixon. (1999). Phytochemicals in soybeans: Their potential health benefits. Inform 10:315–321.
- G C Yen, P D Duh, and C L Tsai. (1993). Relationship between antioxidant activity and maturity of peanut hulls. J Agric Food Chem 41:67–70.
- V Yermilov, J Rubio, M Becchi, M D Friesen, B Pignatelli, and H Ohshima. (1995). Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis 16:2045–2050.