Abstract
The present study determined the anti-inflammatory activity and related mechanisms of scopoletin. It was found that scopoletin significantly inhibited croton oil-induced mouse ear edema. Scopoletin could also decrease the vascular dye leakage induced by topical application of croton oil, consistent with the reduced myeloperoxidase (MPO) activity and polymorphonuclear (PMN) infiltration. The inhibitor of nitric oxide (NO) synthase l-Nω -nitro-arginine (l-NN, 10 mg/kg) attenuated croton oil-induced ear edema by 56.5%. However, pretreatment of l-NN did not affect the inhibitory effects of scopoletin (100 mg/kg) on mouse ear edema, which suggested that scopoletin exerted anti-inflammatory activities in an endothelial NO-independent manner. Further research revealed that scopoletin reduced the overproduction of PGE2 and TNF-α in croton oil-treated mouse ears. Scopoletin was also shown to attenuate the hind paw edema induced by carrageenan in mice, and lower the MPO activity and malondialdehyde (MDA) level in paw tissues. These findings imply that the anti-inflammatory activities of scopoletin involve inhibition of eicosanoid biosynthesis, cell influx, and peroxidation.
Introduction
Gout results from intra-articular deposition of monosodium urate (MSU) crystals in individuals with elevated serum concentrations of uric acid (CitationAgudelo & Wise, 2001; CitationRoubenoff, 1990). The treatment of hyperuricemia associated with gout entails the use of xanthine oxidase inhibitors to block the biosynthesis of uric acid from purine, and uricosuric drugs for patients who poorly excrete uric acid. Concurrently, anti-inflammatory agents are always demanded to relieve the inflammatory symptoms of gout. Such a therapy strategy based on drug combinations is generally effective, whereas limitations lie on the adverse events and high economic load for patients. It would be attractive and promising for gout therapy if one compound has both hypouricemic and anti-inflammatory activities.
Scopoletin is a coumarin isolated from the stems of Erycibe obtusifolia Benth (Convolvulaceae), which was usually used for rheumatic arthritis therapy in Traditional Chinese Medicine. It has been reported to possess hypotensive (CitationOjewole & Adesina, 1983), xanthine oxidase inhibitory (CitationChang et al., 1994), and anti-oxidant activities (CitationShaw et al., 2003; CitationYun et al., 2001). Data from our laboratory demonstrated that scopoletin was effective against experimental hyperuricemia models through the inhibition of uric acid production and uricosuric mechanisms (CitationDing et al., 2005). Additionally, in vitro and in vivo preliminary investigations had shown that scopoletin was capable of inhibiting the production of PGE2, TNF-α, IL-1β and IL-6 as well as nitric oxide synthesis in RAW 264.7 cells (CitationKim et al., 2004; CitationKang et al., 1999), and reducing TPA- and ethyl phenylpropiolate-induced ear edema in mice (CitationMuschietti et al., 2001; CitationFarah & Samuelsson, 1992). These findings suggest that scopoletin deserved to be further studied as a potential remedy for the treatment of gout.
Despite the previous work on scopoletin, the anti-inflammatory characteristics of scopoletin, and especially the involved mechanisms, are still poorly understood. This study was thus designed to evaluate the in vivo anti-inflammatory activities of scopoletin in two typical inflammatory models and to explore the possible mechanisms involved, from the view of cell infiltration, proinflammatory cytokine production, and antioxidant activity.
Materials and Methods
Animals
Male ICR mice (20∼ 25 g) were purchased from the animal center of China Pharmaceutical University and were housed in plastic cages. They were allowed one week to adapt to environment before experiments. Animals were maintained on a 12 h light/dark cycle in an air-conditioned room and given standard chow and water ad libitum. All animal experiments were conducted under the ethical regulations for animal care and use of China Pharmaceutical University.
Chemicals and reagents
Scopoletin (purity > 98%) was isolated from the stems of Erycibe obtusifolia Benth, which was purchased from Kunming City, Yunan Province of China in Jan 15, 2004 and was authenticated by Professor Zhengtao Wang. The voucher specimen was deposited in the Department of Pharmacology of Chinese Materia Medica, China Pharmaceutical University. Indomethacin, prednisolone and l-Nω -nitro-arginine (l-NN) were purchased from Sigma Chemicals (St. Louis, MO). Carrageenan was the product of Liaoning Institute of Materia Medica (Shengyang, China). All other chemicals were of the highest analytic grade available. Scopoletin, indomethacin, and prednisolone were suspended in 0.8% CMC-Na solution freshly before use.
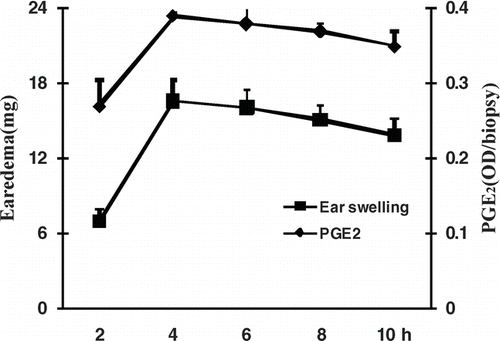
Time course of ear edema and PGE2 levels in mouse dermatitis induced by croton oil
Cutaneous inflammation was induced on the right ears of mice by topical application of 75 μ g/ear of croton oil in 15 μ l acetone (CitationSosa et al., 2001). The left ears, used as controls, received only the vehicle. Five groups of mice were killed by cervical dislocation at different times (2, 4, 6, 8, 10 h) after induction of dermatitis. Ear disks of 8 mm diameter were removed from each ear and the weights were determined. The edema was represented as the difference of weights between the disks from right and left ears. PGE2 concentrations in ear lobes were measured essentially following the previously described procedures (CitationLiu et al., 2003). In brief, a biopsy was homogenized in 1 ml of normal saline. After 1 h, the homogenate was centrifuged. The supernatant (0.3 ml) was mixed with 2 ml of 0.5 N methanol followed by isomerization in a water bath at 50°C for 20 min. The mixture was then diluted to 5 ml with methanol, after which the PGE2 level was measured colorimetrically at 278 nm. The time course curve for edema and PGE2 level were plotted.
Ear vascular permeability in mouse dermatitis induced by croton oil
To determine the vascular permeability of ears, mice were intravenously injected with Evans blue (100 mg/kg) into tail veins 30 min before topical application of croton oil (CitationLiu et al., 2003). At 4 h after the application, the animals were sacrificed. Disks of 8 mm diameter were removed from each ear and then transferred to a polycarbonate tube. They were hydrolyzed with 1 ml of 1.2 N potassium hydroxide solution at 37°C for 24 h. The hydrolysates were neutralized and deproteinized with 4.5 ml of 0.6 N phosphoric acid/acetone mixture (5/13 solution). The mixture was shaken at room temperature for 30 min and then centrifuged for 30 min at 1,000 rpm. The optical density of the extracted Evans blue dye was colorimetrically measured at 610 nm (CitationLloret & Moreno, 1995).
MPO activity, PGE2 and TNF-α level in mouse dermatitis induced by croton oil
At 4 h after the topical application of croton oil, the animals were sacrificed and the right ear lobes of the mice were taken. They were chopped and suspended in 0.75 ml of 80 mM sodium phosphate buffer (pH 5.4). Then, they were homogenized for 5 seconds at 20,000 g. The homogenates were centrifuged at 15,000 g for 5 min and the supernatants were collected and frozen at −80°C until analysis (CitationBradley et al., 1982). MPO (a marker enzyme of PMN infiltration) activity was determined colorimetrically using a commercial kit (MPO Test Kit, Nanjing Jiancheng Bioengineering Insititute, Jiangsu Province, China). PGE2 concentrations were determined as mentioned above. The concentrations of TNF-α were analyzed in duplicate by a quantitative sandwich ELISA (Boster Biological Technology Ltd, Hubei Province, China) according to the manufacturer's instruction.
Paw edema induced by carrageenan in mice
Inflammatory edema was induced on the right hind paws by subplantar injection of carrageenan (1% w/v in saline, 40 μ l) (CitationSiqueira et al., 2003). The thickness of the injected paws was measured before and at 1, 3, and 5 h after induction of inflammation, using a micrometer. The paw swelling was expressed as a thickness increase in relation to the initial values.
To determine MPO activity and malondialdehyde (MDA, an indicator of lipid peroxidation) level in inflamed paws, the animals were sacrificed and the right paws were removed at 3 h after carrageenan injection. The skin was removed and the paws soaked in 1 ml of 15% trichloroacetic acid for 3 h (4°C), then homogenized with normal saline. After centrifugation, MPO and MDA levels in the supernatants were measured colorimetrically using commercial kits (MPO, MDA Test Kit, Nanjing Jiancheng Bioengineering Institute, Jiangsu Province, China).
Statistical analysis
All values in the figures and text were expressed as mean ± standard error of the mean (SEM). Data sets were examined by one- or two-way analysis of variance, and individual group means were then compared with Student's unpaired t-test. A P-value of less than 0.05 was considered significant.
Results
Time course of ear edema and PGE2 levels in mouse dermatitis induced by croton oil
To determine the best time point for the mouse dermatitis model induced by croton oil, a time course study up to 10 h was conducted. Both ear edema and PGE2 levels peaked at 4 h and then decreased gradually after the application of croton oil. The time point at 4 h was therefore selected for observation in the following experiments ().
Effects of scopoletin on ear edema in mouse dermatitis induced by croton oil
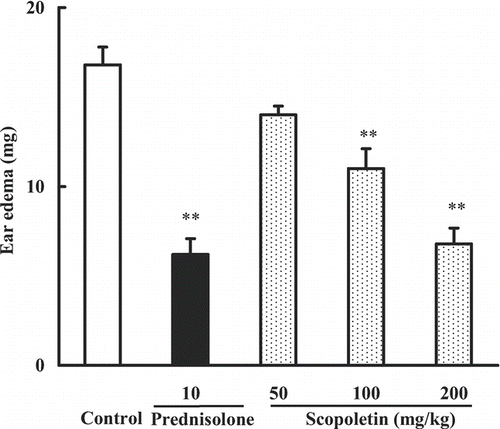
The potential effects of scopoletin against the mouse dermatitis model induced by croton oil were firstly evaluated by measuring the ear edema. Mice were randomly divided into five groups including control, scopoletin (50, 100, 200 mg/kg) and prednisolone (10 mg/kg) groups. Scopoletin and prednisolone were administered intraperitoneally 30 min before application of croton oil. Ear edema was determined at 4 h after the induction of the dermatitis. The results are illustrated in . The ear edema in the control group was 16.8 ± 1.0 mg. Scopoletin (50, 100, 200 mg/kg) reduced the edematous response in a dose-dependent manner with inhibition percentages of 16.7, 34.6, and 59.6%, respectively. The inhibition percentage of prednisolone (10 mg/kg) was 68.2%.
Figure 2. Effects of scopoletin and prednisolone on croton oil-induced mouse ear edema. Values were means ± SEM of 8 mice. Statistically significant difference with respect to control was expressed as **P < 0.01.
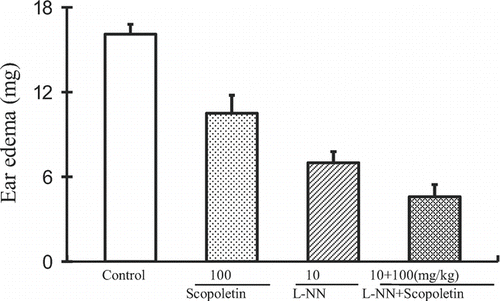
l-NN, an inhibitor of NO synthase, was used to test whether the anti-inflammatory effect of scopoletin was NO-dependent. Mice were randomly divided into four groups including control, scopoletin (100 mg/kg), l-NN (10 mg/kg) and l-NN (10 mg/kg) plus scopoletin (100 mg/kg) groups. Scopoletin and l-NN were intraperitoneally administered 30 and 60 min before the application of croton oil, respectively. Ear edema was determined at 4 h after the induction of the dermatitis. As shown in , scopoletin (100 mg/kg) and l-NN (10 mg/kg) alone attenuated the ear edema by 34.8 and 56.5%, respectively. Combination of scopoletin with l-NN (10 mg/kg) demonstrated no inhibitory ratio change of scopoletin on ear edema (from 34.8 to 34.3%), which suggested that scopoletin inhibited croton oil-induced ear edema in an endothelial NO-independent manner.
Ear vascular permeability in mouse dermatitis induced by croton oil
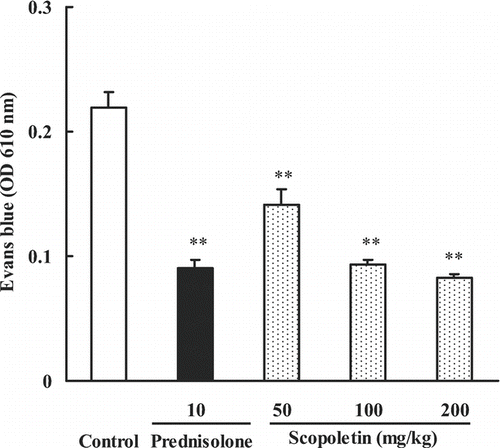
The possible effect of scopoletin on inhibiting the vascular permeability enhancement induced by inflammation was determined by a method of Evans blue leakage. Mice were randomly divided into five groups including control, scopoletin (50, 100, 200 mg/kg) and prednisolone (10 mg/kg) groups. Scopoletin and prednisolone were administered intraperitoneally 30 min before the topical application of croton oil. As shown in , croton oil increased ear vascular permeability evidenced by increased Evans blue leakage. Scopoletin significantly reduced the dye leakage at all test doses and in a dose-dependent way, producing a 35.6 and 62.1% inhibition at doses of 50 and 200 mg/kg, respectively. Prednisolone (10 mg/kg) produced a 58.9% inhibition.
MPO activity, PGE2 and TNF-α level in mouse dermatitis induced by croton oil
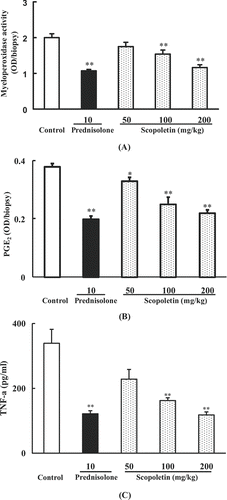
To further investigate the anti-inflammatory mechanisms of scopoletin, the MPO activity, PGE2 and TNF-α level were evaluated in the mouse dermatitis model induced by croton oil. Mice were randomly divided into five groups including control, scopoletin (50, 100, 200 mg/kg) and prednisolone (10 mg/kg) groups. Scopoletin and prednisolone were administered intraperitoneally 30 min before application of croton oil. Results showed that MPO activity in croton oil-treated ears increased significantly. Scopoletin (50, 100, 200 mg/kg) and prednisolone (10 mg/kg) pretreatment markedly lowered the enzyme activities (). Additionally, scopoletin (100, 200 mg/kg) and prednisolone (10 mg/kg) remarkably decreased PGE2 and TNF-α levels of ears ( and ).
Paw edema induced by carrageenan in mice
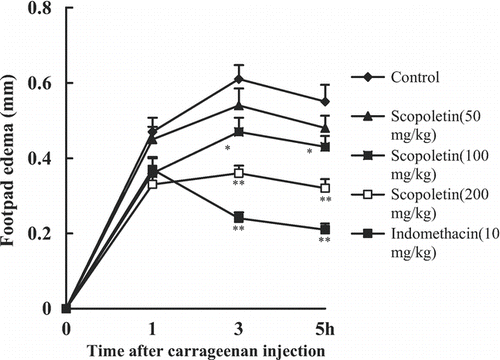
The carrageenan-induced paw edema model, another frequently applied inflammatory model, was also conducted for further evaluation of the anti-inflammmatory effect of scopoletin. Mice were randomly divided into five groups including control, scopoletin (50, 100, 200 mg/kg) and indomethacin (10 mg/kg) groups. Scopoletin and indomethacin were administered intraperitoneally 30 min before carrageenan injection. A control group received the vehicle only. The subplantar injection of carrageenan into mouse hind paw elicited an acute inflammation (swelling and erythema). The maximal swelling appeared at 3 h after carrageenan injection. Scopoletin (100, 200 mg/kg) and indomethacin (10 mg/kg) significantly reduced the paw swelling at 3 and 5 h after injection of carrageenan ().
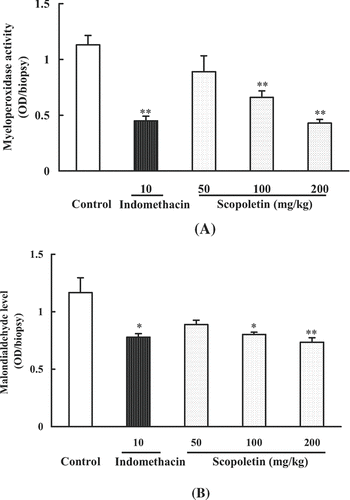
The influence of scopoletin on the MPO activity and MDA level was also determined in the carrageenan induced paw edema mouse model. Mice were randomly divided into five groups including control, scopoletin (50, 100, 200 mg/kg) and indomethacin (10 mg/kg) groups. Scopoletin and indomethacin were intraperitoneally administered 30 min before carrageenan injection. MPO activities and MDA (an indicative of lipid peroxidation) levels in paw tissues were determined at 3 h after carrageenan injection. As shown in and B, MPO activities and MDA levels were significantly reduced by scopoletin (100, 200 mg/kg) and indomethacin (10 mg/kg) treatments.
Discussion
Croton oil-induced dermatitis is an irritant reaction (IR), which is characterized as a non-immunological inflammatory response that appears rapidly after a single application of chemical on the skin (CitationFlint et al., 2000). Since the severest edema occurred at 4 h after croton oil challenge evidenced from a time-course study, the potential effect of scopoletin was evaluated at this time point. Scopoletin exhibited a significant inhibitory effect on MPO activity, indicating that scopoletin could prevent PMN infiltration in croton oil-treated ears (CitationBradley et al., 1982). Furthermore, scopoletin significantly inhibited Evans blue leakage in croton oil-treated ears at all test doses, suggesting that the abnormally high capillary permeability was partially restored, leading to less infiltration of PMN and other inflammatory cells.
In order to elucidate the underlying mechanisms, the influences of scopoletin on NO and PGE2 pathways and on tumor necrosis factor, were further studied. l-NN, an inhibitor of NO synthase, was used either alone or in combination with scopoletin to determine whether the anti-inflammatory effect of scopoletin was endothelial NO-dependent. We found that l-NN (10 mg/kg) and scopoletin (100 mg/kg) alone attenuated croton oil-induced ear edema by 56.5 and 34.8%, respectively. Concurrent injections of scopoletin (100 mg/kg) and l-NN (10 mg/kg) obtained a 34.3% inhibition effect. Presumably, if scopoletin acted in a NO-dependent way, concurrent injection of NOS inhibitor should result in a lower inhibition ratio because the NO production was preliminarily blocked. However, no significant difference was observed when the respective inhibition ratio was compared, suggesting that scopoletin may act in an endothelial NO-independent manner, at least in this croton oil-induced ear edema model. Previous reports revealed that prostaglandins as a vasodilator may play an important role in the development of edema induced by croton oil in the mouse ear (CitationBlazso & Gabor, 1994). Our results showed that the PEG2 reached the highest level at 4 h after the croton oil challenge, and scopoletin at doses over 100 mg/kg could significantly inhibit its production. PGE2 as a vasodilator may potentiate plasma leakage and hence edema formation (CitationWilliams & Peck, 1977), especially at the inflammation site where there is damaged endothelium that is usually caused by the proinflammatory cytokines. Tumor necrosis factor has been proven to be a critical mediator in IR in terms of increasing leucocyte adhesion (CitationPiguet et al., 1991; CitationMorita et al., 1995) and disrupting intercellular junctions of postcapillary venular endothelium (CitationWong et al., 1999). In the present study we demonstrated an overproduction of TNF-α in the homogenate of the croton oil-treated mouse ears, further supporting the pivotal role of TNF-α in the IR process. Scopoletin at doses over 100 mg/kg can significantly inhibit the TNF-α overproduction. In combination with the determined effect on PGE2, these findings help us to postulate that the anti-inflammatory activity of scopoletin on the croton oil-induced model may originate predominantly from its inhibitory effect on PGE2 and TNF-α overproduction.
The carrageenan-induced paw edema model, another frequently applied inflammatory model, was also introduced into the present study for further evaluating the anti-inflammmatory effect of scopoletin. A significant alleviating effect of scopoletin at doses over 100 mg/kg on the paw edema was observed from 3–5 h after carrageenan injection. It has been previously reported that the onset of carrageenan-induced local inflammation was linked to neutrophil infiltration, the production of neutrophil-derived free radicals such as hydrogen peroxide, superoxide, and hydroxyl radical, as well as to the release of other neutrophil-derived mediators (CitationBradley et al., 1982; CitationOyanagui, 1976). MPO activity as a PMN infiltration indicator and MDA level as the end product of oxidative stress were investigated in this study. It was found that scopoletin at doses over 100 mg/kg could significantly inhibit the MPO activity and reduce the MDA level in the paw homogenates. Thus, inhibition of neutrophil infiltration and free radical production might be the important mechanisms underlying the anti-inflammatory activity of scopoletin.
In summary, scopoletin possesses a remarkable anti-inflammatory activity in both croton oil- and carrageenan-induced inflammatory models, possibly originating from its inhibitory activities on PGE2 and TNF-α overproduction, and neutrophil infiltration. In combination with our published work, in which a significant hypouricemic effect was observed for scopoletin on the oxonate-induced hyperuricemic mouse model, this coumarin can be prospectively developed as a drug candidate for the gout therapy.
Acknowledgements
This study was partially supported by the Open Grant of Jiangsu Key Laboratory for Molecular and Medical Biotechnology (No. 164070304301) and the National Natural Science Foundation of China (No. 30672471).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- CA Agudelo, and CM Wise. (2001). Gout: Diagnosis, pathogenesis, and clinical manifestations. Curr Opin Rheumatol 13:234–239.
- G Blazso, and M Gabor. (1994). Anti-edematous action of some H1-receptor antagonists. Agents and Actions 42:13–18.
- PP Bradley, DA Pribat, RD Christensen, and G Rothstein. (1982). Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209.
- WS Chang, YH Chang, FJ Lu, and HC Chiang. (1994). Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res 14:501–506.
- ZQ Ding, Y Dai, and ZT Wang. (2005). Hypouricemic action of scopoletin arising from xanthine oxidase inhibition and uricosuric activity. Planta Med 71:183–185.
- MH Farah, and G Samuelsson. (1992). Pharmacologically active phenylpropanoids from Senra incana. Planta Med 58:14–18.
- MS Flint, DB Miller, and SS Tinkle. (2000). Restraint-induced modulation of allergic and irritant contact dermatitis in male and female B6.129 mice. Brain Behav Immun 14:256–269.
- TH Kang, HO Rae, SJ Jeong, JC Yoo, BM Choi, CD Jun, HT Chunq, T Mivamoto, R Hiquchi, and YC Kim. (1999). Scopoletin: An inducible nitric oxide synthesis inhibitory active constituent from Artemisia feddei. Planta Med 65:400–403.
- HJ Kim, SI Jang, YJ Kim, HT Chung, YG Yun, TH Kang, OS Jeong, and YC Kim. (2004). Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia 75:261–266.
- BL Liu, YY Dai, N Tang, FL Zhang, and C Wang. (2003). Anti-inflammatory activity of chloroform extract of Evodia rutaecarpa on experimental colitis in mice. Pharmacol Clin Chin Mat Med 19:16–19.
- S Lloret, and JJ Moreno. (1995). Effects of an anti-inflammatory peptide (antiflammin 2) on cell influx, eicosanoid biosynthesis and edema formation by arachidonic acid and tetradecanoyl phorbol dermal application. Biochem Pharmacol 50:347–353.
- Y Morita, MG Clemens, LS Miller, U Rangan, S Kondo, M Miyasaka, T Yoshikawa, and GB Bulklev. (1995). Reactive oxidants mediate TNF-alpha-induced leukocyte adhesion to rat mesenteric venular endothelium. Am J Physiol 269:H1833–H1842.
- L Muschietti, S Gorzalczany, G Ferraro, C Acevedo, and V Martino. (2001). Phenolic compounds with anti-inflammatory activity from Eupatorium buniifolium. Planta Med 67:743–744.
- JA Ojewole, and SK Adesina. (1983). Mechanism of the hypotensive effect of scopoletin isolated from the fruit of Tetrapleura tetraptera. Planta Med 49:46–50.
- Y Oyanagui. (1976). Participation of superoxide anions at the prostaglandin phase of carrageenan foot-edema. Biochem Pharmacol 25:1465–1472.
- PF Piguet, GE Grau, C Hauser, and P Vassalli. (1991). Tumor necrosis factor is a critical mediator in hapten-induced irritant and contact hypersensitivity reactions. J Exp Med 173:673–679.
- R Roubenoff. (1990). Gout and hyperuricemia. Rheum Dis Clin North Am 16:539–550.
- CY Shaw, CH Chen, CC Hsu, CC Chen, and YC Tsai. (2003). Antioxidant properties of scopoletin isolated from Sinomonium acutum. Phytother Res 17:823–825.
- JM Siqueira junior, RR Peters, AJ de Brum Fernandes, and RM Riberiro do Valle. (2003). Effects of valeryl salicylate, a COX-1 inhibitor, on models of acute inflammation in mice. Pharmacol Res 48:437–443.
- S Sosa, A Tubaro, U Kastner, S Glasl, J Jurenitsch, and R Della Loggia. (2001). Topical anti-inflammatory activity of a new germacrane derivative from Achillea pannonica. Planta Med 67:654–658.
- TJ Williams, and MJ Peck. (1977). Role of prostaglandin-mediated vasodilation in inflammation. Nature 270:530–532.
- RK Wong, AL Baldwin, and RL Heimark. (1999). Cadherin-5 redistribution at sites of TNF-α and IFN-γ induced permeability in mesenteric venules. Am J Physiol 276:H736–H748.
- BS Yun, IK Lee, IJ Ryoo, and ID Yoo. (2001). Coumarins with monoamine oxidase inhibitory activity and antioxidative coumarino-lignans from Hibiscus syriacus. J Nat Prod 64:1238–1240.