Abstract
The present work examined the effects of the n-butanol extract from the leaves of Kalanchoe crenata (Andrews) Haworth (Crassulaceae) on rat blood pressure and guinea pig papillary muscle contraction and action potential. When administered intravenously at doses of 2, 5, and 10 mg/kg, the n-butanol extract of K. crenata leaves induced a significant transient fall in blood pressure and significantly reduced the cardiac rate for about 10 min. Concomitantly, the extract significantly increased the PR, QRS, and QT intervals of the electrocardiogram (ECG). At concentrations of 300 and 1,000 μ g/ml, the n-butanol extract was found to increase the amplitude of electrical contraction of papillary muscle by 137.6 and 223.0%, respectively. When tested on the ventricular myocardiac cell action potential, the extract (100 μ g/ml) significantly and time dependently delayed the repolarization without affecting the amplitude. Indeed, the 10%, 60%, and 90% repolarization times were respectively increased by 131.6%, 78.6%, and 52.7%. The bradycardic effect of the n-butanol extract may result from the increase of the PR, QRS, and QT intervals which are in accord with the delay in action potential repolarization observed in in vitro studies. The data obtained in the in vitro studies suggest that K. crenata possess potassium channel blockade properties that may account for its cardiac properties.
Introduction
Kalanchoe crenata (Andrews) Haworth (Crassulaceae) is an ornamental plant commonly known as “never die” or “Dog's liver”. This plant is widely used in traditional medicine in the treatment of inflammation, earache, headache, asthma, palpitation, abdominal pain, convulsion, and general debility (CitationSofowora, 1993). Phytochemical investigations have shown the presence of alkaloids and saponins in the aqueous and alcohol extracts of K. crenata leaves (CitationSofowora, 1993) and lectins in the juice from fresh leaves (CitationAdinike & Eretan, 2004). The n-butanol extract from the dry leaves of K. crenata has been shown to be rich in phenolic compounds, flavonoids, and saponins (CitationNguelefack et al., 2006a). Recent work's have shown the analgesic, anticonvulsant, anti-inflammatory, and spasmolytic properties of extracts from K. crenata leaves (CitationNguelefack et al., 2004, Citation2006a, Citation2006b; CitationDimo et al., 2006). From these studies it was suggested that the n-butanol extract may be acting at least partially as a calcium antagonist. It was then thought, consistent with the use of K. crenata leaves in the traditional medicine for the treatment of palpitation, this extract may have some cardiovascular properties.
The present work was therefore undertaken to evaluate the cardiovascular properties of the n-butanol extract of the leaves of Kalanchoe crenata. The extract was tested on rat blood pressure and heart rate, and on guinea pig papillary muscle contraction and action potential.
Materials and Methods
Preparation of the plant extract
Fresh leaves of K. crenata were harvested in Dschang (West Cameroon) in September 2001 and authenticated at the National Herbarium Yaounde, Cameroon where a voucher specimen has been deposited under number 50103/YA. The powder (780 g) obtained from the sun-dried and ground leaves was macerated in a mixture of methanol/methylene chloride (1:1) for 72 h with occasional stirring. The mixture was filtrated and the filtrate was concentrated to dryness to give 110.8 g of extract. This extract (104 g) was suspended in distilled water and successively extracted with hexane, methylene chloride, ethyl acetate, and n-butanol. The concentration of the n-butanol fraction afforded 22.38 g of extract. For in vitro studies, the extract was prepared at the concentration of 100 mg/ml in a mixture of 20% of DMSO and 10% of Tween 20 but administered in such a way that none of the final concentration of vehicle exceeded 0.25%. For in vivo studies, the extract was prepared at a concentration of 10 mg/ml in a mixture of 3% DMSO and 2% Tween 20 and further diluted 2 and 5 times. This last mixture was shown to have no effect on blood pressure and heart rate within 35 min.
Animals
Guinea pigs of either sex, aged between 12 and 16 weeks and weighing about 350 g, were obtained from the animal house of the Institute of Pharmacology and Toxicology of the Technical University of Munich. They were used for in vitro studies on papillary muscle contraction and action potential. Male Wistar rats of the same age but weighing 180 to 200 g obtained from the animal house of the Faculty of Science, University of Yaoundé I, were used for in vivo studies. The animals were housed in colony cages and had free access to standard laboratory diet and water. The ethics committee of the Cameroon Ministry of Scientific Research and Technology which has adopted the guidelines established by the European Union on Animal Care and Experimentation (CEE Council 86/609), approved all experimental procedures.
Effect of extract on blood pressure, heart rate and ECG
The rats (18) were anesthetized using an intraperitoneal injection of urethane (1 g/kg). Arterial blood pressure was measured from the left carotid artery via an arterial cannula connected to a pressure transducer (SS13L, Biopac, France). The recorded signal was transferred to an acquisition system (MP35, Biopac) coupled with a computer. The animals were allowed to stabilize for at least 30 min before administration of the test substance (CitationDimo et al., 2003). The K. crenata n-butanol extract was injected at doses of 2, 5, and 10 mg/kg via the cannula inserted into the right femoral vein. The heart frequency of the animals was determined by spectral analysis of the blood pressure signal. The electrocardiograph electrodes (SSL29L, Biopac) were placed on the same animals in such a way that blood pressure, heart rate and ECG were recorded concomitantly. The signals were visualized and analyzed by means of a computer program (BSL PRO 3.7, Biopac).
Effect of extract on electrical contraction of the guinea pig papillary muscle
The guinea pigs (15) were sacrificed by cervical dislocation. The heart was quickly isolated, immersed in a cold Krebs-Henseleit solution and rapidly dissected. The papillary muscle of the right ventricle was isolated, suspended vertically under a resting tension of 400 mg, in a 50 ml organ bath containing a modified Krebs-Hesenleit physiological solution of the following composition (mM): NaCl 114.9; KCl 1.22; CaCl2 3.2; NaHCO3 24.9; KH2PO4 1.18; MgCl2 1.18; glucose 11. The physiological solution maintained at 35°C was continuously gassed with 95% O2 and 5% CO2. The organ was stimulated (Fa. Tönnies, Freiburg, Germany) with an electrical intensity approximately 10% above the threshold at the frequency of 1 Hz. Isometric contraction curves were monitored on a digital oscilloscope and recorded on a chart recorder by the means of an inductive force transducer (Hottinger Baldwin Messtechnik, Darmstadt, Germany). The organ was equilibrated for 40 min and the solution changed at 20 min (CitationDongmo et al., 2007). Cumulative concentrations of n-butanol extract (10–1,000 μ g/ml) or nifedipine (10–3,000 nM) were then added to the medium.
The organ was exposed to each concentration of n-butanol extract for 5 min while the effect of each nifedipine concentration was observed for 15 min. The amplitude of contraction at the end of the effect of each concentration was expressed as a percentage of initial amplitude taken as 100%.
Action potential of guinea pig ventricular cell
The guinea pigs (10) were sacrificed and the papillary muscle dissected as described above and suspended horizontally in an organ bath containing a modified Krebs-Hesenleit physiological solution maintained at 35°C and continuously gassed with 95% O2 and 5% CO2. The muscle was slightly stretched and stimulated at a frequency of 0.1 Hz with electrical intensity about 10% above the threshold. Transmembrane potentials were recorded in the absence and in the presence of n-butanol extract at the concentration of 100 μ g/ml, using conventional fly microelectrodes filled with 3 M KCl and resistance ranging 10 to 20 MΩ. Recorded action potentials were visualized and analyzed with an analogue-digital converter computer program (Fa. MFK, Niedernhausen, Germany) (CitationSontia, 2005).
Statistical analysis
Data are expressed as mean ± SEM. Significant differences among the mean values of multiple groups were evaluated by ANOVA repeated-measures followed by Tukey as post hoc test except in action potential parameters analysis where ordinary ANOVA was used with Dunn as post hoc test.
Results
Effect of the extract on blood pressure and heart rate
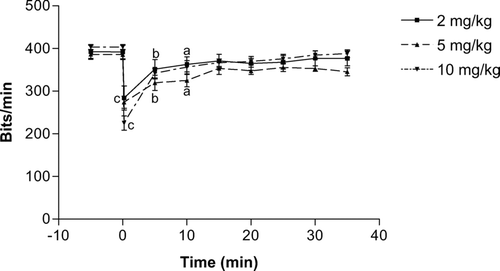
As shown in , the slow administration of the n-butanol extract of K. crenata resulted in a significant transient fall in blood pressure that lasted for 4 min. At the dose of 10 mg/kg the mean arterial blood pressure decreased from 112 to 56.63 mm Hg, representing a drop of 49.4%. Doses of 2 and 5 mg/kg induced similar falls of 44.6% and 52.7%, respectively.
Figure 1. Time-dependent effects of the n-butanol extract of the leaves of Kalanchoe crenata on the mean arterial blood pressure (MABP) of anesthetized normotensive rats. n = 5, cp < 0.001 compared to initial value (0 min).
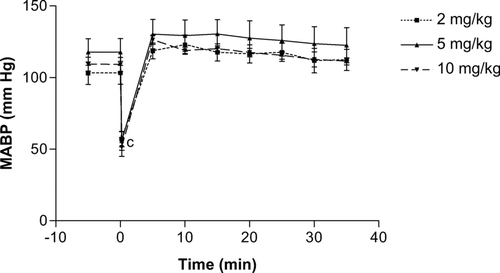
The n-butanol extract of K. crenata also induced a dose-dependent fall in heart rate. The maximum fall obtained immediately after the intravenous administration of the extract was 27.6% (from 392.3 to 284.0 bits/min), 28.9% (385.6 to 273.9 bits/min), and 40.9% (403.2 to 238.2 bits/min) at respective doses of 2, 5, and 10 mg/kg. The heart frequency was still significantly low, as compared to initial values, 10 min after administration of the extract at the doses of 5 and 10 mg/kg. Independently of the dose, the heart rate had not completely recovered after 30 min ().
Effect of extract on ECG
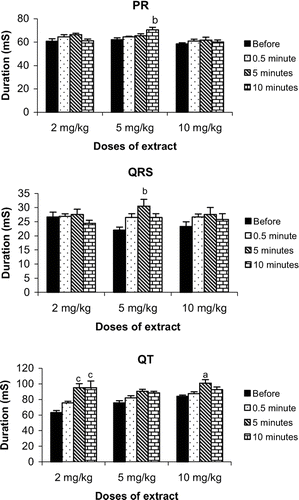
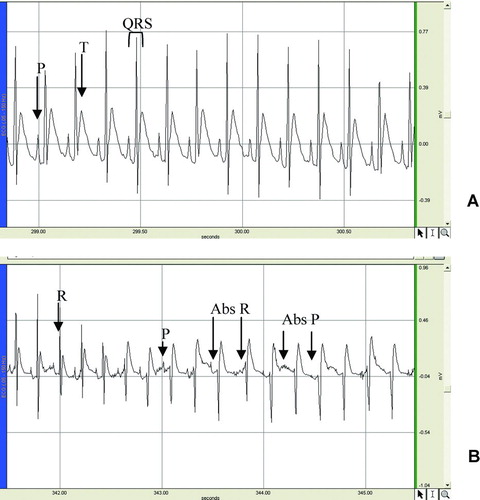
The intravenous administration of the n-butanol extract of K. crenata leaves induced a modification of the electrocardiogram curve marked by the disappearance of the P wave and a transient reduction of the QRS amplitude, due to the absence of the R wave (). Another important fact is that the disappearance of the R wave preceded that of the P wave. These transient effects appeared 30 s after extract administration and lasted for 10 s. The analysis of the ECG waves shows that the extract significantly increased PR interval and QRS duration. In animals receiving 5 mg/kg, the PR interval increased by 12.1% (from 62.0 to 75.5 mS) 10 min after extract administration. At this same dose, the extract increased the QRS duration by 38.6% (from 22.0 to 30.5 mS). The extract also increased the QT interval. In animals treated with 2 mg/kg, this interval increased by 50.5% (from 63.1 to 95.0 mS) 5 and 10 minutes after extract administration. The QT interval was 90.5 ± 2.6 mS and 100.8 ± 4.7 mS in rats treated with 5 and 10 mg/kg, respectively ().
Figure 3. Effects of the n-butanol extract of the leaves of Kalanchoe crenata on the electrocardiogram of anesthetized rat. (A) control, (B) electrocardiogram recorded 30 seconds after extract (10 mg/kg) administration showing the progressive disappearance of P and R waves as well as the reduction of heart frequency. Abs R = absence of R wave; Abs P = absence of P wave.
Effect of the extract on the electrically evoked contractions of papillary muscle
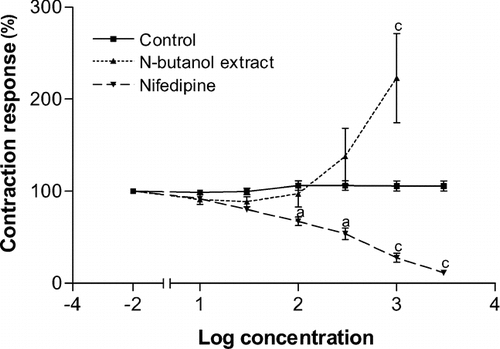
The n-butanol extract was further tested on the contraction of the papillary muscle evoked by electrical stimulation. As shown in , the lower concentrations (10–100 μ g/ml) induced slight non-significant reduction of the amplitude of contraction. Higher concentrations rather increased the contraction amplitude. At concentrations of 300 and 1,000 μ g/ml, the extract increased the amplitude by 137.6 and 223.0%, respectively, compared to the initial amplitude (100%). Nifedipine induced a concentration-dependent decrease in this electrical contraction. The maximum decrease of 88.63% was achieved at a cumulative concentration of 3 μ M.
Effect of the extract on the ventricular cell action potential
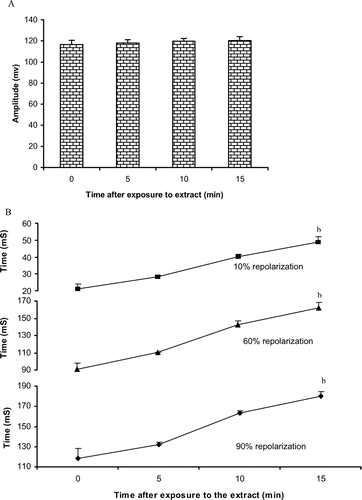
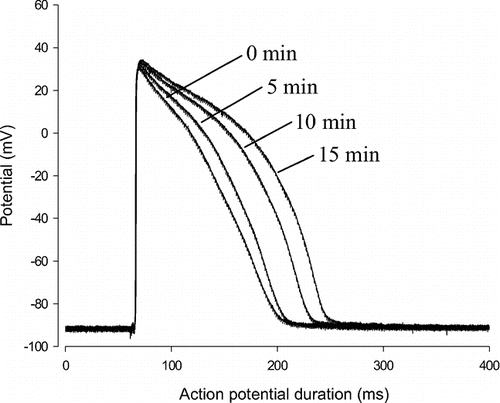
When tested on the action potential of the ventricular muscle cells, the n-butanol extract induced a time-dependent increase in the duration of action potential. This increase becomes significant 15 min after exposition to the drug. At this moment, the 10%, 60%, and 90% repolarization time increased by 131.6% (21.1 to 48.8 mS), 78.6% (90.48 to 161.7 mS) and 52.7% (118.1 to 180.2 mS), respectively. This was equal to an increasing time of 27.75, 71.3, and 62.2 mS, respectively at 10%, 60%, and 90% repolarization. Exposition of the organ to the extract did not significantly affect the amplitude of action potential ( and ). These changes disappeared after washout.
Discussion
This study investigated the effect of the n-butanol extract of Kalanchoe crenata leaves on the blood pressure, electrocardiogram, papillary muscle contraction and cardiac cell action potential. The intravenous administration of the extract in normotensive anesthetized rats induced a blood pressure lowering effect accompanied by a reduction of heart rate. The hypotensive effect lasted 4 min when the heart rate was still significantly low. These results suggested that the hypotensive effect of the extract may be due to its bradycardiac effect. The fall in blood pressure induced by the extract might stimulate the baro-reflexes. Catecholamines are then released to cause a transient rise in pressure due to a vasoconstriction.
The electrocardiogram analysis showed an increase in PR, QRS, and QT intervals and the transient disappearance of the P and R waves. The P wave indicates the depolarization of the auricles. The absence of this wave may be due to the absence of the depolarization of the sinoatrial node (SA). It could be thought that the disappearance of the R wave is the consequence of the absence of atrial depolarization but it was observed that the absence of R preceded that of P. These results suggest that the extract may affect both the SA and the atrioventricular node (AV). Pacemaker depolarization can be caused by either increasing inward current or decreasing outward current (CitationBers, 2002). The K. crenata extract seems to inhibit the depolarization of SA and AV. This suggests that either the extract decreases inward current (INa or ICa) or increases the outward current (IK) or even both. If these were the main mechanisms of the extract, it would have reduced the action potential amplitude and/or duration. But when tested on the ventricular myocardiac cell action potential, the extract did not affect the amplitude and instead increased the duration. Thus, the extract neither depresses the inward current nor increases the outwards. More experiments are needed to understand how the K. crenata n-butanol extract could affect the activities of SA and AV.
It was observed that extract increased the PR interval and QRS duration, suggesting that it could be able to delay the impulse conduction from the atrium to the His bundle and in the myocardium. As stated by CitationVasconcelos et al. (2006), the electrical wave is slowed down in the heart when the myocardium length constant (λ) is shortened or when the propagated action potential amplitude is reduced, or even in both circumstances. As the amplitude of the action potential of the cells treated with the plant extract remained relatively unchanged, we can assume that the extract did not affect the amplitude of propagated impulses. Thus, it may shorten the length constant which is theoretically given by the following equation:
(CitationJack et al., 1975), where Rm stands for the membrane resistance, Ri for the intracellular pathway resistance and a for the fiber radius. Therefore, the reduction of λ would be related either to the decrease of Rm or to the increase of Ri. Accordingly, it has been demonstrated that the increase of Ri induced a delay in the impulse conduction (CitationJalife et al., 1989; CitationFujino et al., 1991, CitationCascio et al., 2001). According to CitationVasconcelos et al. (2006), the reduction in Rm depends on the opening of potassium K1-type channels which leads to the shortening of the QT interval. In the present study the intravenous administration of the extract instead increased the QT interval. It can then be concluded that the extract did not reduce the Rm. Thus, the remaining hypothesis is the increase of the Ri. It has been demonstrated that the increase in free intracellular calcium concentration increases the Ri (Xiao & De Mello, 2001). This may be likely the mechanism of action of K. crenata extract since it significantly increased the force of contraction of papillary muscle which closely depends on the intracellular calcium concentration.
The increase of the QT interval observed was in accordance with the in vitro test on cell action potential that showed the ability of the extract to delay the ventricular repolarization. These findings suggest that the K. crenata extract may slow the potassium outward current without affecting the K1-type channels. These effects are characteristic of class III anti-arrhythmic substances which are K+ channel blockers (CitationValenzuela et al., 1995). The prolongation of the action potential duration could lead to important calcium influx which may explain the positive inotropic effect of the extract. This likely anti-arrhythmic activity of the n-butanol extract is in accordance with the traditional use of K. crenata against palpitation (CitationSofowora, 1993).
The presence of flavonoids in the n-butanol extract of K. crenata may account for its cardiovascular activity. This group of secondary plant metabolites widely occurring in the vegetable kingdom has been shown to display a remarkable array of biochemical and pharmacological actions, including cardiovascular effects (CitationKar et al., 2006; CitationPerez-Vizcaino et al., 2006). In fact, it was found that hesperetin, a flavonoid isolated from orange, has an antagonist effect on cardiac potassium channels (CitationScholz et al., 2007).
In conclusion, n-butanol extract of the leaves of K. crenata significantly reduced blood pressure and heart rate in normotensive anesthetized rat. The bradycardiac effect was related to an increase in PR, QRS, and QT intervals. Accordingly, the long-lasting QT interval was due to a delay in myocardiac cell repolarization. This extract may have K+ channel blocker property which may at least partially be responsible for it cardiac effects.
Acknowledgements
The authors are very grateful to the DAAD and the TWAS for the respective award no. A/04/30012 and the grant no. 03-460/RG/BIO/AF/AC allocated, which gave us the opportunity to carry out this work at the Institute of Pharmacology and Toxicology of the Technical University of Munich in Germany and in the Laboratory of Animal Physiology at the University of Yaounde I in Cameroon.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- K Adinike, and OB Eretan. (2004). Purification and partial characterization of lectin from the fresh leaves of Kalanchoe crenata (And.) Haw. J Biochem Mol Biol 37:229–233.
- MD Bers. (2002). Calcium and cardiac rhythms physiological and pathological. Circulation Res 90:14–17.
- WE Cascio, H Yang, TA Johnson, BJ Muller-Borer, and JJ Lemasters. (2001). Electrical properties and conduction in reperfused papillary muscle. Circulation Res 89:741–743.
- T Dimo, TB Nguelefack, PV Tan, MP Yewah, E Dongo, SV Rakotonirina, A Kamanyi, and M Bopelet. (2003). Possible mechanisms of action of the neutral extract from Bidens pilosa L. leaves on the cardiovascular system of anesthetized rats. Phytother Res 17:1135–1139.
- T Dimo, LA Fotio, TB Nguelefack, EA Asongalem, and P Kamtchouing. (2006). Antiinflammatory activities of extracts from Kalanchoe crenata Andr. (Crassulaceae). Indian J Pharmacol 38:115–119.
- AB Dongmo, AGB Azebaze, TB Nguelefack, BM Ouahouo, B Sontia, M Meyer, AE Nkengfack, A Kamanyi, and W Vierling. (2007). Vasodilator effect of the extracts and some coumarins from the stem bark of Mamea africana (Guttiferae). J Ethnopharmacol 111:329–334.
- T Fujino, N Takahashi, and M Ito. (1991). Arrhythmogenesis of changes in cellular ionic environments: Ischemia-induced increase in intracellular resistance and slow conduction. Rinsho Byori 39:793–800.
- JJB Jack, D Noble, and RW Tsein. (1975): Electric current flow in excitable cells. Oxford, Clarendon Press, p. 502.
- J Jalife, S Sicouri, M Delmar, and DC Michaels. (1989). Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am J Physiol 257:H179–H189.
- P Kar, D Laight, and KM Shaw. (2006). Cummings MH flavonoid-rich grapeseed extracts: A new approach in high cardiovascular risk patients?. Int J Clin Pract 60:1484–1492.
- TB Nguelefack, LA Fotio, P Watcho, S Wansi, T. Dimo, and A Kamanyi. (2004). Analgesic activities of aqueous and ethanolic extracts of the leaves of Kalanchoe crenata (Crassulaceae). Phytother Res 18:385–388.
- TB Nguelefack, P Nana, AD Atsamo, T Dimo, P Watcho, AB Dongmo, LA Tapondjou, D Njamen, SL Wansi, and A Kamanyi. (2006a). Analgesic ant anticonvulsant effects of extracts from the leaves of Kalanchoe crenata (Andrews) Haworth (Crassulaceae). J Ethnopharmacol 106:70–75.
- TB Nguelefack, B Sontia, AB Dongmo, T Dimo, A Kamanyi, and W Vierling. (2006b). Spasmolytic effects of extracts from Kalanchoe crenata Andrews (Crassulaceae) leaves. Pharmacologyonline 1:30–39.
- F Perez-Vizcaino, J Duarte, and R Andriantsitohaina. (2006). Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radical Res 40:1054–1065.
- EP Scholz, E Zitron, C Kiesecker, D Thomas, S Kathöfer, J Kreuzer, A Bauer, HA Katus, A Remppis, CA Karle, and J Greten. (2007). Orange flavonoid hesperetin modulates cardiac hERG potassium channel via binding to amino acid F656. Nutr Metab Cardiovasc Dis 17:629–698.
- A Sofowora. (1993): Medicinal plants and traditional medicine in Africa, 2nd ed. Ibadan, Nigeria, Polygraphie Ventures Ltd, pp. 207–209.
- B Sontia. (2005): Influence of plant extracts (Vitex cienkowskii and Daniella oliveri) and physiological ions on vascular smooth muscles and electrophysiological properties of the heart muscle. Dissertation zum Erwerb des Doktorgrades der Humanbiologie an der Medizinischen Fakultät der Ludwig-Maximilians-Universität zu München. 146P.
- C Valenzuela, J Tamargo, E Delpón, and O Pérez. (1995). Antiarrhythmic agents. Ars Pharm 36:507–526.
- CML Vasconcelos, MS Araujo, and EA Conde-Garcia. (2006). Electrophysiological effects of the aqueous extract of Averrhoa carambola L. leaves on the guinea pig heart. Phytomedicine 13:501–508.
- RP Xiao, and WC De Mello. (1991). Intracellular resistance in rat papillary muscle: Interaction between cyclic AMP and calcium. J Cardiovascular Pharmacol 17:754–760.