Abstract
The ethanol extract of dried fruits of Embelia ribes Burm (Myrsinaceae) was evaluated for protection against isoproterenol (ISO)-induced myocardial infarction in albino rats. The cardiotoxicity induced by ISO (5.25 and 8.5 mg/kg, s.c., for two consecutive days) was indicated by a significant increase (P < 0.01) in heart rate and systolic blood pressure, elevated levels of lactate dehydrogenase (LDH) and creatine kinase (CK) in serum, with increased thiobarbituric acid reactive substances (TBARS), and reduced glutathione (GSH), superoxide dismutase (SOD) and catalase (CAT) levels in heart homogenates, as compared to normal healthy control rats. Microscopical examination (histopathology) was also performed on the myocardial tissue. Pretreatment with ethanol Embelia ribes extract (200 mg/kg, p.o., 40 days) significantly (P < 0.01) decreased the elevated levels of LDH and CK in serum and myocardial TBARS and increased the reduced levels of GSH, SOD and CAT in heart homogenates in ISO-induced myocardial infarction in albino rats. Histopathological observation revealed a marked protection by the extract in myocardial necrotic damage. The results of our study, for the first time, provide clear evidence that ethanol Embelia ribes extract treatment enhances the antioxidant defense against ISO-induced myocardial infarction in rats and exhibit cardioprotective properties.
Introduction
Embelia ribes Burm (Myrsinaceae), commonly known as Vidanga, is a large woody climbing shrub and is widely distributed throughout India. The fruit is bitter in taste, good appetizer, cures tumors, ascites, bronchitis, jaundice, and mental disorders (CitationKirthikar & Basu, 1987). Fruits contain a quinone derivative embelin (3-undecyl 2,5-dihydroxy, 1,4-benzoquinone), an alkaloid christembine (CitationTyagi et al., 1978) and a volatile oil vilangin; its chemical composition is 2,5-dihydroxy-4-undecyl-3,6-benzoquinone (CitationRao & Venkateswaralu, 1961). Previous studies have shown that Embelia ribes has antihyperglycemic (CitationTripathi, 1979), antidiabetic (CitationBhandari et al., 2002), anthelmintic (CitationHordegen et al., 2006) and antioxidant activity (CitationBhandari et al., 2007). The objective of present study was to assess the protective effect of ethanol extract of Embelia ribes against ISO-induced myocardial infarction in albino rats, which has not been reported before.
Materials and Methods
Chemicals
ISO was obtained from Sigma Chemical (St. Louis, MO, USA). All other chemicals used were of analytical grade. Double distilled water was used for all biochemical assays.
Plant material
The dried fruits of Embelia ribes burm were purchased from the local market, New Delhi, India in October, 2005 and the drug was authenticated by the Department of Botany, Faculty of Science, Hamdard University, New Delhi, India where a voucher sample has been deposited (voucher specimen no. UB 2).
The dried and coarsely powdered drug (100 g) was packed in a Soxhlet apparatus and was subjected to extraction with ethanol over 72 h. The filtrate was evaporated under vacuum drier and brown mass residue obtained was stored at 4°C for further use. The average yield of the ethanol Embelia ribes extract was approximately 7.9%. For experimental study, the weighed amount of ethanol Embelia ribes extract (200 mg/kg) was dissolved in 1% Tween 80 in normal saline and administered to albino rats by the oral route.
Animals
Wistar albino rats of either sex, weighing between 200–250 g, were procured from the Central Animal House Facility, Hamdard University, New Delhi and acclimatized under standard laboratory conditions at 25° ± 2°C, 50 ± 15% RH and normal photoperiod (12 h light:dark cycle) for 7 days. Commercial rat pellet diet (Nav Maharastra Chakan Oil Mills Ltd, Delhi, India) and water were provided ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Hamdard University, New Delhi, which is registered with Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, India (Registration no. 173/CPCSEA, dated 28 January, 2000).
Anti-myocardial infarction activity
Albino rats of either sex were randomly divided into four groups of ten animals each and treated as follows:
Group I, Normal healthy control–rats received only 1% Tween 80 in normal saline; Group II, Pathogenic control–rats received only ISO (5.25 and 8.5 mg/kg) for two consecutive days (CitationVogel, 2002); Group III, Rats received ethanol Embelia ribes extract (200 mg/kg, p.o.) for 40 days followed by ISO (5.25 and 8.5 mg/kg) administration for two consecutive days; Group IV, Rats received gliclazide (25 mg/kg, p.o.) for 40 days followed by ISO (5.25 and 8.5 mg/kg) administration for two consecutive days.
The hemodynamic parameters, viz., heart rate and blood pressure were recorded on 0 day and the 43rd day of all the groups of albino rats, using a non-invasive method of tail cuff plethysmography, with a LE 5001 pressuremeter (LETICA Scientific Instruments, USA) (CitationTomlinson et al., 1991).
After the experimental period, blood samples were withdrawn from the retro-orbital plexus using the micro-capillary technique (CitationSorg & Buckner, 1964) from all the groups of overnight fasted rats, and serum was separated by centrifugation for biochemical estimation of marker enzymes LDH (CitationLum et al., 1974) and CK (CitationRosalki, 1967).
After blood collection, all animals were sacrificed by cervical dislocation and hearts were dissected out and immediately frozen in liquid nitrogen. Six hearts from each group were chosen for lipid peroxide (CitationOhkawa et al., 1979), glutathione (CitationSedlak & Lindsay, 1968) superoxide dismutase (Marklund, 1974), and catalase (CitationAebi, 1974) measurements. The protein content was determined via the method described by CitationLowry et al. (1951).
The remaining four myocardial tissues from all the groups were subjected to histopathological studies as described by CitationLuna et al. (1960). The tissues were fixed using 10% formalin, routinely processed and embedded in paraffin wax. Paraffin section (5 μm) were cut on glass slides and stained with hematoxylin and eosin (H & E) after dewaxing, and examined under a light microscope by a pathologist blinded to the groups studied.
Statistical analysis
All data were expressed as mean ± s.e.m. All the groups of data were analyzed by one-way analysis of variance followed by Dunnett t-test using Graphpad Prism 3.0 (Graphpad software; San Diego, CA). P < 0.01 values were considered as statistically significant.
Results
Rats trusted with isoproterenol (group II) showed a significant (P < 0.01) increase in heart rate, systolic blood pressure, and LDH and CK levels in serum, as compared to normal control rats, i.e., group I. Pretreatment with ethanol ER extract (group III) and gliclazide (group IV) showed a significant (P < 0.01) decrease in heart rate and LDH and CK levels in serum as compared to pathogenic control rats (group II). However, no significant (P > 0.05) changes in systolic BP were observed with ethanol ER extract and gliclazide treated rats ().
Table 1. Effect of ethanol Embelia ribes extract administration on heart rate, systolic blood pressure, serum LDH and serum CK levels.
In isoproterenol-induced myocardial damage, the levels of lipid peroxides were increased and GSH, SOD and CAT levels were decreased (group II) significantly (P < 0.01), as compared to levels in normal healthy control rats (group I). Further, ethanol ER extract (group III) and gliclazide (group IV) treatment showed a significant decrease (P < 0.01) in myocardial TBARS levels and a significant increase in the myocardial GSH, SOD and CAT levels as compared to pathogenic control rats (group II) ().
Table 2. Effect of ethanol Embelia ribes extract administration on myocardial TBARS, GSH, SOD and CAT levels.
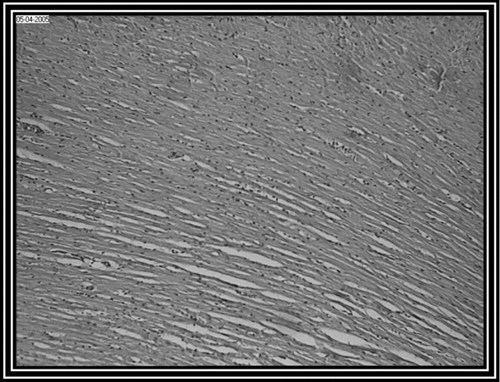
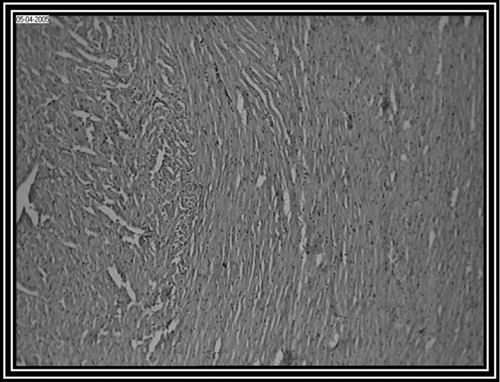
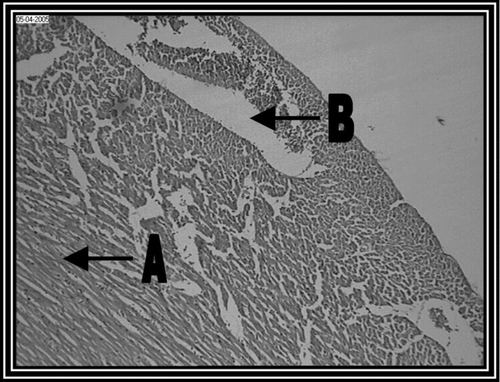
Photomicrograph of control group revealed a normal architecture with regular morphology of myocardial cell membrane (). There was confluent necrosis of cardiac muscle fibre with infiltration of red blood cells was observed in the pathogenic control group (). Photomicrograph of ethanol Embelia ribes extract treatment group (i.e., group III) showed normal myocardial fibres with no inflammation (). Photomicrograph of gliclazide treated group (i.e., group IV) showed normal myocardial fibres ().
Figure 1. Normal healthy control group (i.e., group I) rat heart section, showing normal myocardial fibres (10 x).
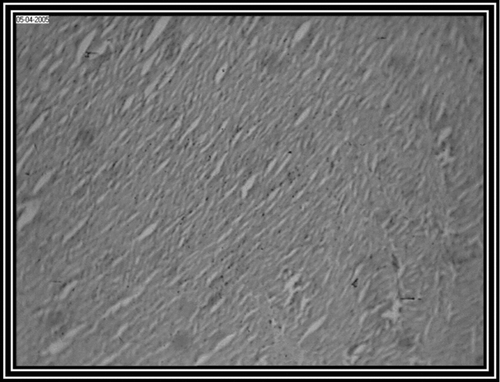
Figure 2. Pathogenic control group (i.e., group II) rat heart section, showing, (A) Marked inflammatory infiltrate with (B) edema (10 x).
Discussion
The present study was designed to assess the cardiovascular response in animals subjected to isoproterenol (ISO)-induced infarction and its protection by ethanol Embelia ribes (ER) extract treatment. Our study demonstrated an increase in heart rate and systolic BP in ISO treated animals. A notable decrease in heart rate was seen in animals treated with ethanol ER extract and gliclazide, although we could not observe any significant decrease in the systolic BP. The induction of myocardial infarction in experimental animals by ISO is probably due to action on the sarcolemmal membrane, stimulation of adenylate cyclase, activation of Na+ and Ca+ channels, exaggerated Ca+ inflow and energy consumption leading to cellular death (CitationMilei et al., 1978). Further, a significant (P < 0.01) increase in serum LDH and serum CK levels occurred in ISO-treated rats. An increase in the levels of serum LDH and CPK indicate cardiac muscular damage which could be due to the leakage of enzymes from the heart as a result of ISO-induced myocardial necrosis (CitationSheela & Shyamaladevi, 2000). Free radicals generated by ISO initiate lipid peroxidation of the membrane bound polyunsaturated fatty acids, leading to impairment of the membrane structural and functional integrity (CitationAjitha & Rajnarayana, 2001). This concurs with the present findings wherein the levels of TBARS were found to be significantly (P < 0.01) increased in animals subjected to ISO exposure. Due to this increased lipid peroxidation, glutathione levels are lowered (CitationFlohe, 1989). Further, the ISO-treated animals showed decreased activity of the key antioxidants SOD, CAT and GSH, which play an important role in scavenging the toxic intermediates of incomplete oxidation. The decrease in the activity of these antioxidants can lead to an excess availability of the superoxide anion (O2−) and hydrogen peroxide in biological systems, which in turn generate hydroxyl radicals resulting in initiation and propagation of lipid peroxidation.
In the present study, pretreatment with ethanol ER extract significantly (P < 0.01) reduced the levels of serum LDH and serum CPK suggesting cardioprotective potential of Embelia ribes. Further, the levels of myocardial lipid peroxides were reduced and GSH, SOD and CAT were increased significantly (P < 0.01), thereby, enhancing the endogenous myocardial antioxidant levels. Furthermore, the results of test drug were comparable to gliclazide, a standard positive control.
Furthermore, histopathological observations revealed that the ethanol ER extract prevented the degeneration of myofibriller tissue and leucocytic infiltration in myocardial necrosis. In conclusion, the results obtained from our study indicate that the rats pretreated with ethanol ER extract are significantly protected from myocardial damage caused by isoproterenol.
Acknowledgment
The research project was supported by project grant to Dr. Uma Bhandari by University Grants Commission, New Delhi, India.
References
- M Aebi. (1974): Catalase. In: HU Bergmeyer. ed., Methods of Enzymatic Analysis. Second ed., vol. 2, Chemic Academic Press, New York, pp. 673–685.
- M Ajitha, and K Rajnarayana. (2001). Role of oxygen free radicals in human disease. Indian Drugs 38:545–554.
- U Bhandari, R Kanojia, and KK Pillai. (2002). Effect of ethanol extract of Embelia ribes on dyslipidaemia in diabetic rats. Int J Exp Diab Res 3:159–162.
- U Bhandari, N Jain, and KK Pillai. (2007). Further studies on antioxidant potential and protection of β -cells by Embelia ribes in experimental diabetes. Exp Diab Res 2007:1–6.
- L Flohe. (1989): The selenoprotein glutathione peroxidase. In: D Dolphin, R Poulson, O Avramsvic. eds., Glutathione: Chemical, Biochemical and Medical Aspects. New York, John Wiley & Sons, pp. 634–731.
- P Hordegen, J Cabaret, H Hertzberg, W Langhans, and V Maurer. (2006). In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J Ethnopharmacol 108:85–89.
- KR Kirthikar, BD Basu. (1987): Indian Medicinal Plants, Vol. 2, Lalit Mohan Basu, Allahabad, India, p. 1479.
- OH Lowry, NJ Rosebrough, AL Farr, and RJ Randall. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275.
- G Lum, and SR Gambino. (1974). A comparison of serum vs heparinised plasma for routine chemistry tests. Am J Clin Pathol 61:108–113.
- LC Luna. (1960): Manual of Histological Screening Methods of Armed Forces Institute of Pathology. McGraw Hill Book Co., New York, pp. 125.
- SL Marklund. (1985): Pyrogallol autooxidation. In: R. A. Greenwald. Handbook of Methods for Oxygen Radical Research. Boca Raton, CRC Press, London, UK, pp. 243–247.
- J Milei, RG Nunez, and M Rapaport. (1978). Pathogenesis of isoproterenol induced lesions in the rat myocardium. Cardiol 63:139–143.
- H Ohkawa, N Ohishi, and K Yagi. (1979). Assay of lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:355–358.
- CB Rao, and V Venkateswaralu. (1961). Vilangin a new constituent of Embelia ribes. Curr Sci 30:250–260.
- SB Rosalki. (1967). An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med 69:696–705.
- J Sedlak, and RH Lindsay. (1968). Estimation of total, protein bound and non-protein SH groups in tissue with Ellman's reagent. Anal Biochem 25:192–205.
- C Sheela Sasikumar, and CS Shyamaladevi. (2000). Protective effect of abana. A polyherbal formulation on isoproterenol induced myocardial infarction in rats. Indian J Pharmacol 32:198–201.
- DA Sorg, and B Buckner. (1964). A simple method of obtaining venous blood from small laboratory animals. Proc Society Exptl Biol Med 115:1131–1132.
- KC Tomlinson, SM Gardiner, and T Bennett. (1991). Blood pressure measurement. American J Physiol 258 (4):R 852. Pt 2
- SN Tripathi. (1979). Screening of hypoglycemic action in certain indigenous drugs. J Res Indian Med Yoga & Homeopathy 14:159–169.
- RD Tyagi, MK Tyagi, HR Goyal, and K Sharma. (1978). A chemical study on Krmiroga. J Res Indian Med 3:130–132.
- HG Vogel. (2002): Drug Discovery and Evaluation, Pharmacological Assays. 2nd Ed, Springer-Verlag Berlin Heidelberg, New York, p. 191–192.