Abstract
The fruits of Silybum marianum (L.) Gaertn (Compositae) contain silymarin, an isomeric mixture of flavonolignans (silybin, silychristin, and silydianin). Silymarin acts as a strong anti-hepatotoxic. Silybum marianum cell cultures represent an alternate source of flavonolignans, but the yields of these compounds in such cultures are very low. In this study, in vitro cell culture systems of Silybum marianum were used to monitor the effect of elicitation (picloram, jasmonic acid, and light) on cell growth and production of silymarin. The presence of silymarin was established by high performance liquid chromatography and the greatest growth rates dry weight (32 mg) and silymarin content (0.413 mg g− 1 DW) were obtained with 3 mg l− 1 picloram and 2 mg l− 1 jasmonic acid in darkness after 28 days. The light grown cell suspension cultures showed reduced growth in term of dry weight (24 mg) and silymarin content. The data presented in this study demonstrate that media supplemented with 3 mg l− 1 picloram with JA in dark conditions can be useful for production of silymarin and growth in cell suspension cultures of Silybum marianum.
Introduction
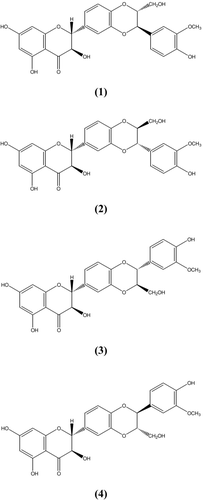
Silymarin is a plant-drived flavonoid isolated from the fruits of the milk thistle, Silybum marianum (L.) Gaertn (Compositae) (CitationKatiyar, 2002). The hydroflavonol taxifolin (TXF) and the flavonolignans silybin (SBN), isosilybin (ISBN), silydianin (SDN), and silychristin (SCN) are usually encompassed by the term of silymarin (SLM) () (CitationSanchez-Sampedro et al., 2005a). The extracts of the fruits have been used for oral treatment of toxic liver damage and for therapy of chronic inflammatory liver disease and liver cirrhosis (CitationSanchez-Sampedro et al., 2005a; CitationSonnenbichler et al., 1999). Their hepatoprotective activity seems to be based on antioxidant properties (CitationAlikaridis et al., 2000). It has recently been shown that SLM may also be beneficial for reducing the chances for developing certain cancers (CitationSanchez-Sampedro et al., 2005b; CitationKatiyar et al., 1997), inhibition of leucotriene production and cholesterol biosynthesis (CitationAlikaridis et al., 2000). Pharmacological studies indicate that SLM is not toxic even at physiological higher doses, which indicates its safe use for the treatment of disease (CitationKatiyar, 2002).
Plants are a tremendous source for discovery of new products of medicinal value for drug development and plant cell culture technologies are possible tools for both studding and producing plant secondary metabolites. Hairy roots, callus and cell suspension culture derived from milk thistle (CitationVanisree et al., 2004) are able to produce SLM (CitationRahnama et al., 2008; CitationSanchez-Sampedro et al., 2005a; CitationTumova et al., 2004), but to a lesser extent than that accumulated in the fruits (CitationSanchez-Sampedro et al., 2005a; CitationAlikaridis et al., 2000; CitationCacho et al., 1999). However, in some cases the manipulation of the components of the medium has substantially increased the production of these compounds (CitationCacho et al., 1999).
Jasmonic acid (JA) and its methyl ester, methyl jasmonate (MJ), have been proposed to be key signaling compounds in the process of elicitation leading to the accumulation of various secondary metabolites (CitationWalker et al., 2002). They have also been reported to play an important role in signal transduction processes (CitationWalker et al., 2002). Light is known to affect the metabolism of various secondary products including diterepenes and sesquiterepenes (CitationFetto-Neto et al., 1995), but such studies have never been applied to S. marianum cultures. We believe that optimization of SLM production in cell suspension culture ultimately would be a consequence of optimizing both conditions for rapid production of SLM and to better understand the metabolic and accumulation of SLM in cell cultures of S. marianum. Therefore, we investigated the influence of 4-amino-3,5,6-trichloropicolinic acid (Pic), JA and white light in cell cultures of S. marianum.
Materials and Methods
Plant material
Dried fruits (Hungarian seeds) of milk thistle were supplied by the Institute of Medical Plants, Iranian Academic Center for Education, Culture and Research (ACECR). The seeds were sterilized by dipping in sodium hypochlorite 2% (w/v), Tween 20, 0.1% (v/v), and rinsed exhaustively with sterile distilled water. The seeds were cultured in hormone-free Murashige and Skoog (MS) medium and incubated in the dark at 26 ± 1°C. Cotyledon, shoots and root segments from 10-day-old seedlings were cultured in Petri dishes containing 25 ml MS medium (CitationCacho et al., 1999) supplemented with 30 g l− 1 sucrose, 1 mg l− 1 2,4-dichlorophenoxyacetic acid (2,4-D) and 0.4 mg l− 1 kinetin (Kin). Calli were developed from explants after 30 days of culture in darkness. The culture growth index was calculated as:
(CitationLindsey, 1985).
The calli from cotyledon segments were subcultured every 4 weeks. Although not shown in this report, previous work demonstrated a different growth index and flavonolignan content in calli culture threatened with different kinds and concentrations of oxin (2,4-dichlorophenoxy acetic acid, naphthalene acetic acid and indole-3-acetic acid and picloram) and cytokinin (kinetin and benzylaminopurin). For this reason we examined media supplemented with different concentrations of picloram (Pic) (1, 2 and 3 mg l− 1) and Kin or BAP (0.4, 0.8 or 1.5 mg l− 1) (CitationHasanloo et al., 2007).
The calli from media supplemented with 3 mg l− 1 Pic and 0.4 mg l− 1 Kin after 90 days were transferred in Petri dishes containing MS medium supplemented with picloram (3 mg l− 1) and Kin (0.4 mg l− 1). All cultures were grown for 30 days in the dark at 25 ± 1°C. Cell suspensions were established from cultures in the same medium and were maintained in 250 ml Erlenmeyer flasks with 50 ml of medium. The suspensions were shaken at 120 rpm in darkness and subcultured after 2 weeks in MS medium were supplemented with Pic (2 or 3 mg l− 1), JA (0 or 2 mg l− 1) and Kin (0.4 mg l− 1). JA was added to the media after autoclaving. These suspensions were shaken at 120 rpm and were kept for 4 weeks. In some experiments, cultures were cultivated only in the dark or only in the light. Each experimental work was carried out in at least three independent subcultures. The growth index and cell viability were determined every week according to CitationChawla (2003), and dry weight of cells was determined after 4 weeks.
Extraction
The calli and the cells from suspension were separated from the medium and the samples were defatted by ethyl acetate. The flavonolignans were extracted from the dried residue with 10 ml of methanol at 40°C for 8 h. The methanol solution was concentrated to a dry residue. The extract was dissolved in 1 ml of methanol and kept at 4°C in darkness (CitationCacho et al., 1999).
Chemicals
Standards of SLM, SBN, TXF, p-coumaric acid, naringenin, were purchaced from Sigma; SCN and SDN from Phytolab; coniferyl alcohol from Aldrich; 2,4-D, Kin, Pic and JA from Sigma; agar and solvents from Merck.
Measurement of Flavonolignans
Spectrophotometric method
Methanol extract (1 ml) was transferred to a 10 ml flask and a 2 ml aliquot of 2,4-dinitriphenilhydrasine-sulfuric acid was added, and then placed in a water bath for 50 min at 50°C. The solution was made up to 10 ml with KOH-methanol 10%. From this flask 1 ml was transferred to a centrifuge tube and a 20-ml aliquot of methanol was added. The mixture was centrifuged. The aqueous phase was then transferred to a 50 ml balloon. It was then diluted to volume (50 ml) with methanol and spectrophotometer (Perkin Elmer, 550 SE) was used with wavelength about 490 nm. For measurement of silymarin, equation 1 was used, using the specific absorbance (A1%1 cm = 537) (CitationHasanloo et al., 2005b).
A = Absorbance, M = Weight of drug.
Determination and quantification of silymarin by HPLC
The amounts of flavonolignans were determined by high-performance liquid chromatography (HPLC) according to CitationHasanloo et al. (2005a) on a Knauer liquid chromatography equipped with a Knauer injector with a 20 μ l loop, a Nucleosil C18 5 μ (250 × 4.6 mm) column, Knauer K2600A UV detector and Chromgate software for peak integration. Mobil phase consisted of the solvents, with a gradient program (). All solvents and chemicals were of HPLC grade (Merck). The elution time and flow rate were 30 min and 1 ml min− 1 and peaks detected at 288 nm.
Table 1. HPLC gradient solvent program.
Identification was achieved by comparison of retention times (Rt) of standards of SCN, SDN, SBN, TXF and a standard mixture of SLM.
Quantification of these metabolites, expressed in mg g− 1 of dry weight, was accomplished using a known concentration of standard and peak areas. A standard solution of SBN was freshly prepared. With dilution of this methanol solution, a series of solutions at different concentrations was prepared and used as standards. The data obtained from the analysis of each solution allowed the plotting of a calibration curve showing a good linearity (correlation coefficient = 0.999).
All experimental analyses were carried out on a minimum of three independent samples for each treatment and three replications of each sample were assayed. Statistical significance was calculated using Duncan test for unpaired data (![]() ≤ 0.05) and ANOVA method was used for comparisons of means. Statistical analysis was made by SAS software (Version 6.2).
≤ 0.05) and ANOVA method was used for comparisons of means. Statistical analysis was made by SAS software (Version 6.2).
Results
In the past 40 years, numerous strategies such as cell line selection, medium optimization and cell immobilization, the use of differentiated cells, elicitation and, more recently, metabolic engineering have been developed to improve the productivity of plant cell culture (CitationShams-Ardakani et al., 2005; CitationVerpoorte et al., 1999). In vitro cultures of milk thistle have been shown to accumulate SLM (CitationAlikaridis et al., 2000; CitationCacho et al., 1999). However, there are fewer reports in the manipulation of the components of the culture medium for increasing these compounds. Therefore, we studied flavonolignans production in the cell suspension culture of S. marianum.
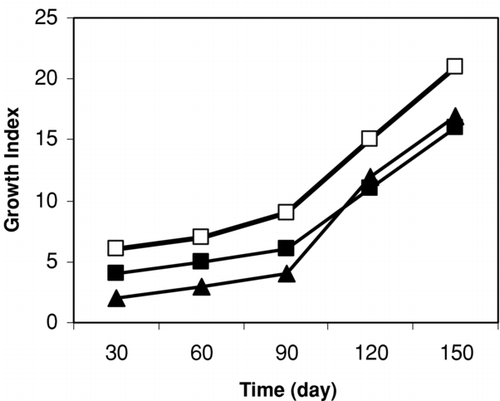
For callus induction, three types of explants (cotyledon, shoot and root segments) of seedlings were used. Cotyledon segment was the best for callus induction, because the growth of the calli seemed to be superior compared to the other tested segments ().
Figure 2. Growth of callus of Silybum marianum from leaf (□), shoot (▪) and root (▵) segments on MS media. Data are the averages of three experiments in triplicate (means ± S.E.).
Picloram (4-amino-3,5,6-trichloropicolinic acid) is not commonly used for plant tissue culture (CitationFurmanowa et al., 1997). It was very interesting that picloram had positive effects on growth and flavonolignan production in calli of Silybum marianum ().
Figure 3. Effect of different treatments on flavonolignan (%) accumulation in calli culture culture of S. marianum. Treatments refer to: Pic (1, 2 or 3 mg l−1), Kin (A) (0.4, 0.8 or 1.5 mg l−1) and: BAP (B) (0.4, 0.8 or 1.5 mg l−1) after 60 (□) and 90 (▪) days. Data are the averages of three experiments; in triplicate (means ± S.E.).
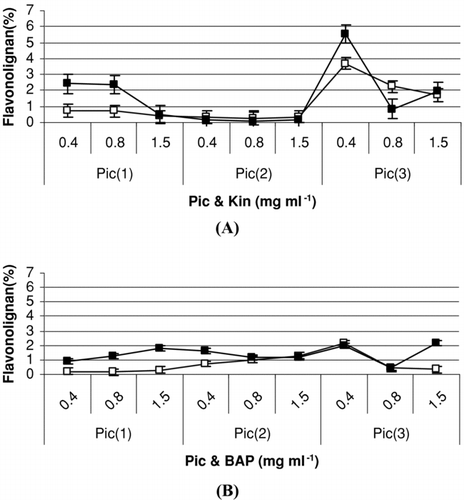
In our observation, after 60 and 90 days, 3 mg l− 1 picloram caused better growth of cultivated callus than 1 or 2 mg l− 1 picloram in MS medium (). The highest growth index of calli was obtained in medium containing picloram (3 mg l− 1) and 0.4 mg l− 1 Kin.
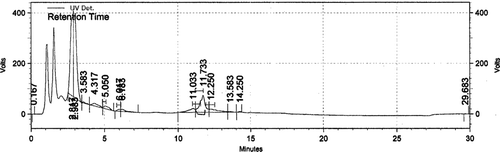
There were eight isolated components: coumaric acid (0.4 mg g− 1 DW), TXF (0.001), SCN (0.007), SDN (0.013), SBNA (0.215), SBNB (0.01), ISBNA (0.072), and ISBNB (0.032) by the HPLC method () from the calli cultured on medium containing Pic (3 mg l− 1) and Kin (0.4 mg l− 1) after 3 months. The amount of SLM was 0.447 mg g− 1 DW. The cell suspension produced similar flavonolignans to those observed on callus cultured on the same medium.
Figure 4. Chromatogram of methanolic extract from callus cultures of S. marianum on MS media supplemented with 3 mg l−1 picloram and 0.4 mg l−1 kinetin.
To determine the effect of treatments on the growth and flavonolignan content of cell suspension of S. marianum, four media (including treatments) (2 or 3 mg l−1 Pic, 0 or 2 mg l−1 JA and 0.4 mg l−1 Kin in dark and light conditions) were prepared. Elicitors inducing JA and its derivatives are known to stimulate production of secondary metabolites in plants (CitationWalker et al., 2002; CitationSans et al., 2000). It has previously been reported that dark conditions influence growth of cell cultures of H. performatum and increase production of hypericin. Therefore, we examined the effects of JA and light or dark on growth and production of SLM in cell suspension cultures of S. marianum. We observed that higher contents of SLM in cell cultures were grown in the dark compared to that of cultures grown in the light in media containing 3 or 2 mg l− 1 Pic and 2 mg l− 1 JA. These results seem to agree with the results of CitationFett-Neto et al. (1995), who reported that light inhibited taxol accumulation in cell culture of Taxus cuspidate. Light-grown cell suspension showed reduced growth in terms of dry weight and the lowest weight was obtained in media supplemented with 2 mg l− 1 Pic without jasmonic acid (). Our results showed that dark-grown suspensions in media were supplemented by 3 mg l− 1 Pic have higher dry weight (32 mg). Three mg l− 1 Pic caused better growth of cell suspension in the dark, but the effect of Pic was not marked on SLM content ().
Figure 5. Effect of different treatments on biomass accumulation (A) and flavonolignan content (%) (B) in cell suspension culture of S. marianum on day 28 under light and dark conditions. Treatments refer to: 1: Pic (3 mg l−1); 2: Pic (3 mg l−1) and JA (2 mg l−1); 3: Pic (2 mg l−1); 4; Pic (2 mg l−1) and JA (2 mg l−1). Data are the averages of three experiments in triplicate (means ± .S.E.).
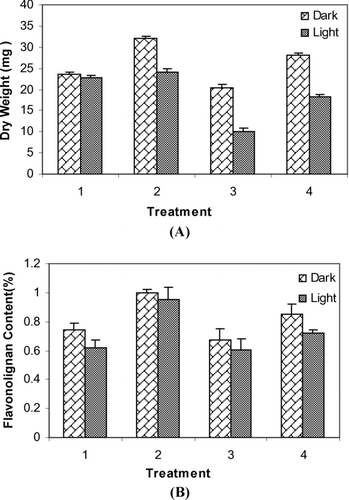
With supplementation of 2 mg l− 1 JA, cell suspension cultures of S. marianum in dark conditions showed an increased biomass production (32 and 24 mg) (). It was observed that treatment with 2 mg l− 1 JA under dark conditions triggered even greater cell growth as compared to the JA-elicited cultures under light conditions. The growth rate of the cells cultured in liquid media has been shown in .
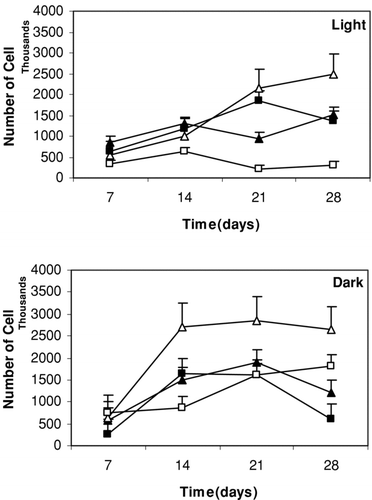
Figure 6. Influence of different treatments on the number of cells in suspension culture of S. marianum on days 7, 14, 21 and 28 under light and dark conditions. Treatments refer to: ▵: Pic (3 mg l−1); ▵: Pic (3 mg l−1) and JA (2 mg l−1); ▪: Pic (2 mg l−1); □: Pic (2 mg l−1) and JA (2 mg l−1). Data are the averages of three experiments in triplicate (means ± S.E.).
The SLM content (1.04 mg g− 1 DW) and dry weight (32 mg g− 1 DW) of dark-grown suspension in media supplemented by 3 mg l− 1 Pic and 2 mg l− 1 JA were higher than media containing 3 mg l− 1 Pic without JA.
Moreover, from the early period of the culture (7 days), the number of cells gradually increased in media supplemented with 3 mg l− 1 Pic, 2 mg l− 1 JA and 0.4 mg l− 1 Kin under dark conditions and reached to its highest level after 1 week (14 days). In the light, the amount of cells increased and reached its highest level after 28 days ().
As shown in , the highest number of cells was obtained in media supplemented with 3 mg l− 1 Pic and 2 mg l− 1 JA after 14 days in darkness and 28 days in light conditions.
Eight components were isolated from the cell suspension cultures of S. marianum in media supplemented by 3 mg l− 1 Pic and 2 mg l− 1 JA under dark conditions. These components were coumaric acid (0.27 mg g− 1 DW), TXF (0.088), SCN (0.006), SDN (0.009), SBNA (0.09), SBNB (1.115), ISBNA (0.065), and ISBNB (0.028) ().
Table 2. Flavonolignan content in cell suspension culture of Silybum marianum grown in media supplemented with 3 mg l− 1 Pic and 2 mg l l− 1 JA in dark conditions. Component were determined and quantified by by HPLC analysis. (Taxifolin, TXF; Silychristin, SCN; Silydianin, SDN; Silybin, SBN; Isosilybin, ISBN and Silymarin (SLM)). Data are the averages of three experiments in triplicate (means± S.E.).
Discussion
It is necessary to evaluate and screen various elicitors with different mechanisms to determine the effects on the production and accumulation of valuable metabolites for pharmaceutical industries by plant cell cultures (CitationShams-Ardakani et al., 2005). Plant cell culture is often an effective system to study the biological significance of bioactive metabolites under in vitro conditions (CitationKetchum et al., 1995; CitationWalker et al., 2002).
Furthermore, optimization of cellular proliferation in suspension cultures is the first step toward establishing large-scale production of these compounds (CitationWalker et al., 2002). For instance, cell suspension cultures of S. marianum have been used to increase production of SLM and these systems might be developed as an alternative system for production of secondary metabolites. The present study examined the influence of dark and light conditions, JA and Pic on the accumulation of SLM in cell suspension cultures of S. marianum.
It was shown that growth and production of SLM in cell suspension cultures of S. marianum were strictly regulated by dark conditions. SLM accumulation in light-grown cell suspensions was lower than that of dark-grown suspensions in media supplemented with JA. It seems that increased SLM content may be attributed to treated cell cultures, which would activate the synthesis of secondary metabolites, agreeing with reported activity by CitationWalker et al. (2002).
A possible effect of light in inhibiting growth might be the changes in metabolism of gibberellins. Reduced amounts of active gibberellins have been found in light-grown tobacco calli and this has been associated with lower growth rates (CitationSeibert & Kadkade, 1980; CitationFetto-Neto et al., 1995). Another possibility is through the down-regulation of key regulatory enzymes responsible for synthesis of mevalonate and its role in cell dividing zones and biosynthesis of gibberellins and cytokinines (CitationFetto-Neto et al., 1995). It also may be due to the stimulation of the biosynthesis of phenolics by light and decrease in activity of various enzymes or low expression of key enzymes and down-regulated catabolism (CitationFetto-Neto et al., 1995).
The effect of Pic has been studied by CitationKetchum et al. (1995), CitationBringi et al. (1995) and CitationFuranowa et al. (1997). In yew tissue culture, according to the results of CitationFuranowa et al. (1997), Pic enhanced the growth of callus. In our observation, 3 mg l− 1 Pic caused better growth and higher accumulation of SLM in cell suspension culture of S. marianum.
The most interesting observation for cell suspension culture of S. marianum was the stimulatory effect of JA on SLM production and growth rate. The cell culture treated with JA and 3 mg l− 1 Pic and incubated in the dark showed increased growth and SLM production as compared to the cultures grown under light conditions. JA in dark conditions played an important role in growth and SLM production in cell suspension cultures of S. marianum. JA showed different effects on dry weight and number of cells in dark or light conditions. A larger dry weight related to cells grown in media supplemented by 3 mg l− 1 Pic and 2 mg l− 1 JA was observed in both light and dark grown suspensions. Jasmonates are possibly signal compounds in the elicitation process leading to de novo transcription and translation and to the biosynthesis of secondary metabolites in plant cell cultures (CitationSans et al., 2000).
The results of these experiments have led to a medium optimized for growth and production of SLM in cell suspension culture of S. marianum and we showed that production of SLM and growth index in cell suspension cultures of S. marianum were strictly regulated by dark conditions and treatment with JA.
Acknowledgements
This work was made possible by financial support from the Agricultural Biotechnology Research Institute of Iran (ABRII).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- F Alikaridis, D Papadakis, K Pantelia, and T Kephalas. (2000). Flavonolignan production from Silybum marianum transformed and untransformed root cultures. Fitotrapia 71:379–384.
- V Bringi, PG Kadkade, CL Prince, BF Schumehi, EJ Schumehi, B Roach, (1995): Enhanced production of taxol and taxanes by cell cultures of Taxus species. United States. WO patent 9317121.
- M Cacho, M Moran, P Corchete, and J Fernandez-Tarrago. (1999). Influence of medium composition on the accumulation of flavonolignans in cultured cells of Silybum marianum (L.) Gaertn. Plant Sci 144:63–68.
- HS Chawla. (2003): Plant Biotechnology. A Practical Approach. Science Pub Inc., Enfield NH. pp.127–129.
- AG Fetto-Neto, JJ Pennington, and F Dicosmo. (1995). Effect of white light on taxol and baccatin III accumulation in cell cultures of Taxus cuspidata Sieband Zucc. Plant Soil 146:584–590.
- M Furanowa, K Glowniak, K Sylowska-Baranek, G Zgorka, and A Jozefczyk. (1997). Effect of picloram and methyl jasmonate on growth and taxane accumulation in cell culture of Taxus × media var. hatfieldii. Plant Cell Tissue Org Cul 49:75–79.
- T Hasanloo, RA Khavari-Nejad, E Majidi, and MR Shams-Ardakani. (2005a). Analysis of flavonolignans in dried fruits of Silybum marianum (L.) Gaertn from Iran. Pak J Biol Sci 8:1778–1782.
- T Hasanloo, RA Khavari-Nejad, E Majidi, SA Ziai, and MR Shams-Ardakani. (2005b). Determination of flavonolignan of dried fruits of Silybum marianum L. Gaertn collected from different areas of Iran by spectrophotometer, TLC and HPLC. J Med Plant 4:25–32.
- T Hasanloo, RA Khavari-Nejad, and E Majidi. (2007). Media optimization for flavonolignans accumulation and growth index of callus culture of Silybum marianum (L.) Gaertn. J. of Plant Sci. Res. 1:7–12.
- SK Katiyar. (2002). Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int J Oncol 21:1213–1222.
- SK Katiyar, NJ Korman, and H Mukhta. (1997). Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst 89:556–66.
- REB Ketchum, DM Gibson, and L Greenspan Gallo. (1995). Media optimization for maximum biomass production in cell culture of Pacific yew. Plant Cell Tiss Org Cul 42:185–193.
- K Lindsey. (1985). Manipulation, by nutrient limitation, of the biosynthetic activity of immobilized cells of Capsicum frutescens Mill. CV. Annual Plantarum 165:126–133.
- H Rahnama, T Hassanloo, MR Shams, and R Sepehrifar. (2008). Silymarin production in hairy root culture of silybum marianum (L.) Gaetn. Iranian J. Biotechnol. 6:113–118.
- MA Sanchez-Sampedro, J Fernandez-Tarago, and P Corchete. (2005a). Yest extract and methyl jasmonate induced silymarin production in cell culture of Silybum marianum L. Gaerth. J Biotechnol 119:60–69.
- MA Sanchez-Sampedro, J Fernandez-Tarrago, and P Corchete. (2005b). Enhanced silymaril accumulation is related to calcium deprivation in cell suspension cultures of Silybum marianum L. Gaertn. Plant Physiol 162:1177–1182.
- MK Sans, XE Hernandez, CE Tonn, and E Guerreio. (2000). Enhancement of tassaric acid production in Tessaria absinthioides cell suspension cultures. Plant Cell Rep 19:821–824.
- M Seibert, and PG Kadkade. (1980): Environmental factors: A. Light. In: EJ Staba. ed., Plant Tissue Culture as a Source of Biochemicals. Boca Raton, CRC Press, pp. 123–141.
- MR Shams-Ardakani, S Hemmati, and A Mohagheghzadeh. (2005). Effect of elicitors on the enhancement of podophylotoyin biosynthesis in suspension cultures of Linum album. Daru 13:56–60.
- J Sonnenbichler, F Scaleram, I Sonnenbichler, and R Weyhenmeyer. (1999). Stimulatory effects of silibinin and silichristin from the milk thistle Silybum marianuna on kidney cells. J Pharmacol Exp Ther 29:1375–1383.
- L Tumova, K Gallova, and J Rimakova. (2004). Silybum marianum. In vitro. Ceska Slov Farmacy 53:135–40.
- M Vanisree, CY Lee, SF Lo, SM Nalawade, CY Lin, and HS Tsay. (2004). Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sinica 45:1–22.
- R Verpoorte, R Van der Heijden, and H JGJ Ten Hoopen. (1999). Metabolic engineering of plant secondary metabolite pathways for the production of fine chemicals. Biotechnol Lett 21:467–79.
- TS Walker, H Pal Bais, and JM Vivanco. (2002). Jasmonic acid-induced hypercin production in cell suspension cultures of Hypericum perforatum L. (St. John's wort). Phytochemistry 60:289–293.