Abstract
Brazilian green propolis, which is used in food and beverages to improve health and to prevent diseases, demostrates antioxidant, antimutagenic, and antimicrobial activities. Most biological activities are thought to be related to the high levels of drupanin, artepillin C, and baccharin, which are compounds also present in Baccharis dracunculifolia D.C. (Asteraceae). Since propolis chemical composition depends on the region and the period of collection, as well as its plant origin, the effect of seasonal variation on the both content of prenylated p-coumaric acids and in vitro antimicrobial activity of Brazilian propolis from four different sites, was performed. The results showed that MIC values ranged from 100 to 300 μg/mL against both Staphylococcus aureus and Kocuria rhizophila, while none of the propolis samples was active against Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. HPLC analysis showed that the content of drupanin, artepillin C, and baccharin varied throughout the year, as well as among the different study sites. Also, it is suggested that Baccharis dracunculifolia is the main botanical source of Brazilian propolis in sites 1 and 2, while in sites 3 and 4, other plant species are also used by bees to produce propolis. All the evaluated propolis samples exhibited similar antibacterial activity, but different contents of prenylated p-coumaric acids throughout the year.
Introduction
Propolis, a resinous substance collected from buds and exudates of certain plants, is the most important “chemical weapon” of honeybees against pathogenic microorganisms. However, the composition of propolis depends on several factors, such as the region, the period of collection, and its plant origin, which determine its chemical diversity (CitationBankova, 2005). Because of the geographical differences, propolis samples from Europe, South America, and Asia have different chemical compositions. Also, it was demonstrated that in Brazil, the propolis collection takes place throughout the year, while the European honeybees collect propolis only in summer (CitationBankova et al., 1998; CitationSousa et al., 2007). Due to the differences in chemical compositions, the biological activities of Brazilian propolis samples from different areas may vary, which creates a serious problem for medical use and quality control of the Brazilian propolis (CitationBankova et al., 2000; CitationLeitão et al., 2004).
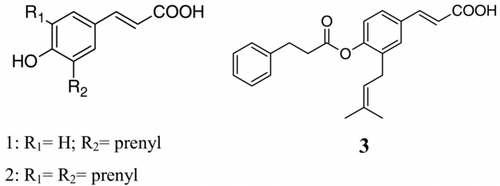
According to literature data, in Brazil, the most important plant source of propolis is Baccharis dracunculifolia D.C. (Asteraceae) (CitationDa Silva Filho et al., 2004). It was recently observed that honeybees (Apis mellifera) bit and chew leaves of B. dracunculifolia, and after processing this material, they use it as propolis, which due to its colour is called green propolis (CitationKumazawa et al., 2003). Several biological activities have been reported for Brazilian green propolis, such as antiulcer (CitationBarros et al., 2007), antioxidant (CitationSimões et al., 2004), anti-inflammatory (CitationReis et al., 2000), antimutagenic (CitationTavares et al., 2006), antifungal (CitationKujumgiev et al., 1999) and antibacterial (CitationLeitão et al., 2004) activities. For this reason, nowadays Brazilian, green propolis is extensively used in food and beverages to improve health and to prevent diseases (CitationKumazawa et al., 2003; CitationLeitão et al., 2004). Some authors state that the biological activities of Brazilian green propolis are mostly due to the high levels of prenylated p-coumaric acids, mainly drupanin, artepillin C, and baccharin, which are also present in B. dracunculifolia (CitationBankova, 2005; CitationSimões et al., 2004; CitationBanskota et al., 2001). Drupanin (1), artepillin C (2), and baccharin (3), the major components of Brazilian green propolis, display important biological activities, such as antitumoral, apoptosis-inducing, immunomodulatory, and antioxidant activities (CitationKonishi et al., 2005; CitationTavares et al., 2006; CitationBanskota et al., 2001).
Since the different geographical regions of Brazil are covered with a conspicuous diversity of plant species and considering that many factors could affect the propolis chemical composition, investigations on the seasonal variations of both chemical composition and biological properties of propolis are important not only for academic interest, but also for the chemical and biological standardization of a particular type of propolis (CitationMissima et al., 2007; CitationBankova et al., 2000). Also, a detailed knowledge of the prenylated p-coumaric acids contents during the year may provide the basis for choosing the most appropriate timing of propolis harvesting in terms of high levels of these important compounds.
Thus, as part of our work on the chemical and biological characterization of Brazilian green propolis (CitationSousa et al., 2007; CitationBarros et al., 2007; CitationSimões et al., 2004) and B. dracunculifolia (CitationLemos et al., 2007; CitationMissima et al., 2007; CitationDa Silva Filho et al., 2004, CitationLeitão et al., 2004), the aim of this study was to evaluate the effect of seasonal variation on the both content of prenylated p-coumaric acids and in vitro antimicrobial activity of Brazilian propolis from four different sites.
Materials and Methods
Propolis material
Colonies of Africanized honey bees (Apis mellifera scutellata) were installed in four different sites of São Paulo and Minas Gerais states, Brazil, in the cities of Capetinga (site 1), Chave da Taquara (site 2), Restinga (site 3) and Franca (site 4). A Langstroth type of hive was used, bearing a brood chamber and honey super. The hives' queens were naturally fecunded and the colonies were not fed during the entire experiment. Propolis was produced using an Apis flora® type cap collector, and the samples were collected from hives between September 2001 and December 2002.
Preparation of propolis ethanol extracts
Dried propolis (20 g) was kept in a freezer for 12 h, ground in a blender, and macerated with aqueous ethanol 70% (100 ml), at room temperature, for 30 days. An aliquot of 10 ml of each extract was concentrated under vacuum, until the complete elimination of ethanol, to furnish semisolid crude ethanol extracts.
Antimicrobial assay
Four bacteria (Staphylococcus aureus ATCC 25923, Kocuria rhizophila ATCC 9341, Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922) and a yeast (Candida albicans ATCC 10231) were used to evaluate the antimicrobial activity of propolis samples. The minimum inhibitory concentration (MIC) values of the crude ethanol extracts were determined in triplicate by using the microdilution broth method (CitationAndrews, 2001). The inoculum was adjusted to each organism to yield a cell concentration of 108 colony forming units (CFU/mL). Standard antibiotics were used as positive controls, and appropriate negative controls were undertaken, as well. The microplates (96-well) were incubated at 37°C for 24 h. After that, 40 μL of 2,3,5-triphenyltetrazolium chloride (0.7%) in aqueous solution were added to indicate the viability of microorganisms (CitationLeverone et al., 1996). The MIC was determined as the lowest concentration of the propolis extract capable of inhibiting microorganism growth.
HPLC analysis
Instrumentations consisted of a Shimadzu (SCL-10Avp, Japan) multisolvent delivery system, Shimadzu SPD-M10Avp photodiode array detector, and an Intel Celeron computer for analytical system control, data collection, and processing. Analytical chromatography of the propolis crude ethanol extracts was carried out using a reversed-phase column CLC-ODS (M) – 4.6 × 250 mm Shimadzu. Propolis samples were dissolved in methanol (1.0 mg/ml) and filtered through a 0.45 μm filter prior to the injection of 20 μL into the HPLC system. A gradient starting with the mixture of 0.8% acetic acid: 0.3% ammonium acetate: 5.0% methanol/water and 25% of acetonitrile, and finishing with 100% of acetonitrile, over 60 m (flow rate 1.0 ml/min), was used to separate the major compounds. The hydroalcoholic extract from B. dracunculifolia leaves was also subjected to HPLC analysis using the same conditions. The spectral data were collected within 60 min over the 280–340 nm range of the absorption spectrum, and the chromatograms were analyzed and plotted at 280 nm.
The prenylated p-coumaric acids (1,3), , were identified by comparison with authentic standards available at the Pharmacognosy Laboratory previously isolated from Brazilian propolis and B. dracunculifolia (CitationDa Silva Filho et al., 2004; CitationSimões et al., 2004, CitationMissima et al., 2007), by comparing UV spectra and considering both the maximum lambda and the relative area obtained with the use of two wavelengths (A280/320). The compounds were quantified by using the internal standard benzophenone by comparing its peak areas with the area of the internal standard.
Results and Discussion
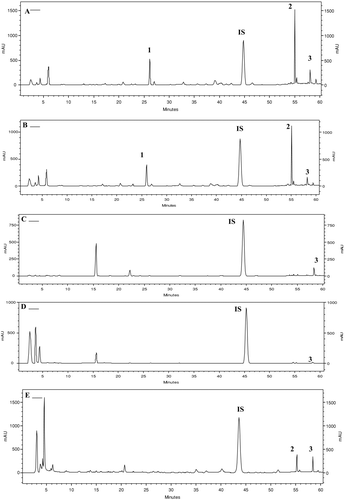
According to literature data (CitationKujumgiev et al., 1999), propolis samples from different geographic origins may have different levels of antimicrobial activity. In the antimicrobial assay, the values of minimum inhibitory concentration ranged from 100 to 300 μg/mL for Gram-positive bacteria. On the other hand, none of the propolis samples showed activity against E. coli, P. aeruginosa, and C. albicans using the undertaken protocol, as shown in . No significant variability in the antimicrobial activity was observed for the sites along the year.
Table 1. Seasonal variations on the prenylated p-coumaric acids (1–3) and antimicrobial activity of different Brazilian propolis samples.
Prenylated p-coumaric acids have been reported to be the most abundant and the most effective compounds in Brazilian green propolis, which possess antitumoral and antioxidant activities related to the high content of drupanin (1), artepillin C (2), and baccharin (3). In this study, it was observed that the content of prenylated p-coumaric acids (1–3) varied throughout the year, as well as among the different sites of propolis collection. The results of the quantitative HPLC analysis () demonstrated the propolis samples from sites 1 and 2 displayed similar HPLC profiles (), which were characterized by the presence of three prenylated p-coumaric acids (1–3) in all samples throughout the year. On the other hand, in sites 3 and 4, drupanin (1) and artepillin C (2) were detected only in propolis samples produced in April and December, while baccharin (3) was detected in all propolis samples. The seasonal variation in the content of prenylated p-coumaric acids may be related to the difference in the preferred regional plants collected by honeybees. Based on the HPLC profiles of these samples, it may be suggested that B. dracunculifolia, the main botanical source of Brazilian green propolis, was visited by the bees in the sites 1 and 2, since identical prenylated p-coumaric acids (1–3) were present in this species, as well as in all analyzed samples from the sites 1 and 2 (). B. dracunculifolia is the predominant botanical source for the Brazilian green propolis production in southeastern Brazil, where the majority of commercialized propolis samples are produced (CitationKumazawa et al., 2003; CitationDa Silva Filho et al., 2004). In sites 3 and 4 low amounts of prenylated p-coumaric acids were found, suggesting that other plant species are also used as a source of propolis by bees. Analysis of the results indicates that the antimicrobial activity of the evaluated Brazilian propolis samples may be related not only to prenylated p-coumaric acids, but also to other constituents, such as flavonoids and terpenes, which are also present in the Brazilian propolis (CitationSousa et al., 2007; CitationBarros et al., 2007; CitationSimões et al., 2004; CitationDa Silva Filho et al., 2004). Overall, all these findings may have economic significance in terms of improving the quality of Brazilian propolis with regard to its pharmaceutical applications. Finally, considering the obtained results, further biological and chemical studies are needed with respect to Brazilian propolis standardization, aiming for practical applications in therapy.
Acknowledgements
The authors are very grateful to the University of Franca for both financial support and transportation arrangements; Abdala Dagher Neto for helping both the installation of hives and collection of the propolis samples; the farm owners for allowing the installation of hives on their properties. We also thank CNPq (Grant # 524689/96-2) and FAPESP (01/14219-7; 04/13005-1) for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- JM Andrews. (2001). Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16.
- V Bankova. (2005). Recent trends and important developments in propolis research. eCAM 2:29–32.
- V Bankova, SL de Castro, and MC Marcucci. (2000). Propolis: Recent advances in chemistry and plant origin. Apidol 31:3–15.
- V Bankova, G Boudourova-Krasteva, S Popov, JM Sforcin, and SRC Funari. (1998). Seasonal variations of the chemical composition of Brazilian propolis. Apidol 29:361–367.
- AH Banskota, Y Tezuka, and S Kadota. (2001). Recent progress in pharmacological research of propolis. Phytother Res 15:561–571.
- MP Barros, JPB Sousa, JK Bastos, and SF Andrade. (2007). Effect of Brazilian green propolis on experimental gastric ulcers in rats. J Ethnopharmacol 110:567–571.
- AA Da Silva Filho, PCP Bueno, LE Gregório, MLA Silva, S Albuquerque, and JK Bastos. (2004). In vitro trypanocidal activity evaluation of crude extract and isolated compounds from Baccharis dracunculifolia D. C. (Asteraceae). J Pharm Pharmacol 56:1195–1199.
- Y Konishi, Y Hitomim, M Yoshida, and E Yoshioka. (2005). Absorption and bioavailability of artepillin C in rats after oral administration. J Agric Food Chem 53:9928–9933.
- A Kujumgiev, I Tsvetkova, Y Serkedjieva, V Bankova, R Christov, and S Popov. (1999). Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol 64:235–240.
- S Kumazawa, M Yoneda, I Shibata, J Kanaeda, T Hamasaka, and T Nakayama. (2003). Direct evidence for the plant origin of Brazilian propolis by the observation of honeybee behavior and phytochemical analysis. Chem Pharm Bull 51:740–742.
- DPS Leitão, AA Da Silva Filho, ACM Polizello, JK Bastos, and ACC Spadaro. (2004). Comparative evaluation of in vitro effects of Brazilian green propolis and Baccharis dracunculifolia extracts on cariogenic factors of Streptococcus mutans. Chem Pharm Bull 27:1834–1839.
- M Lemos, MP Barros, JPB Sousa, AA Da Silva Filho, JK Bastos, and SF Andrade. (2007). Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity. J Pharm Pharmacol 59:603–608.
- MR Leverone, TC Owen, FS Tieder, GJ Stewart, and DU Lim. (1996). Resting-cell dehydrogenase assay measuring a novel water soluble formazan detects catabolic differences among cells. J Microbiol Methods 25:49–55.
- F Missima, AA Da Silva Filho, GA Nunes, PCP Bueno, JPB Sousa, JK Bastos, and JM Sforcin. (2007). Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. J Pharm Pharmacol 59:463–468.
- CMF Reis, JCT Carvalho, LRG Caputo, KCM Patrício, MVJ Barbosa, AL Chieff, and JK Bastos. (2000). Atividade antiinflamatória, antiúlcera gástrica e toxicidade subcrôncia do extrato etanólico de própolis. Braz J Pharmacogn 10:43–52.
- LMC Simões, LE Gregorio, AA Da Silva Filho, ML De Souza, A ECS Azzolini, JK Bastos, and YM Lucisano-Valim. (2004). Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. J Ethnopharmacol 94:59–65.
- JPB Sousa, N AJC Furtado, R Jorge, AEE Soares, and JK Bastos. (2007). Perfis físico-químico e cromatográfico de amostras de própolis produzidas nas microregiões de Franca (SP) e Passos (MG), Brasil. Braz J Pharmagogn 17:85–93.
- DC Tavares, GR Mazzaron Barcelos, LF Silva, CC Chacon Tonin, and JK Bastos. (2006). Propolis induced genotoxicity and antigenotoxicity in Chinese hamster ovary cells. Toxicol in vitro 20:1154–1158.