Abstract
The present study was conducted to isolate the most important bioactive compound from Cinnamomum zeylanicum L. (Lauraceae) bark oil. The plant essential oil was extracted via steam distillation. Cinnamaldehyde was separated using a separating funnel and identified according to Tollen’s test followed by detection on TLC plates in comparison with standard cinnamaldehyde that served as positive control. Moreover, FTIR spectrometry and HPLC analysis were used to confirm the purity and identity of cinnamaldehyde. The isolated material was investigated for its antibacterial activity against six selected pathogenic bacteria. The Gram-positive bacteria were Staphylococcus aureus and Bacillus cereus; Gram-negative bacteria included Escherichia coli, Proteus mirabilis, Klebsiella pneumonia, and Pseudomonas aeruginosa. Cinnamaldehyde at different concentrations (1:1, 1:5, 1:10 and 1:20) was active against all tested bacteria and the highest inhibitory effect was observed against B. cereus (zone of inhibition: 25.3 mm) using the disk diffusion method. The minimal inhibitory concentration (MIC) of cinnamaldehyde was determined using a broth microdilution method in 96-well microtiter plates. MIC values ranged from 31.2 to 125.0 μg/mL. The most promising result was observed against B. cereus, while S. aureus, E. coli, and K. pneumonia ranked next (MIC: 62.5 μg/mL) followed by P. mirabilis and P. aeruginosa with a MIC of 125.0 μg/mL.
Introduction
The medicinal use of plants is widespread all over the world in folk medicine (CitationHernández et al., 2003). Based on this broad use of plants for medicinal purposes, more than 1500 plants have been studied scientifically regarding their phytochemical profile and pharmacological properties (CitationDuarte et al., 2005).
Lauraceae is an economically important family consisting mostly of trees. The genus Cinnamomum comprises ∼250 species that are distributed in Asia and Australia (CitationJayaprakasha et al., 2003). In Iraq, Cinnamomum zeylanicum L. bark oil has been reported to show several medicinal properties: analgesic, antiseptic, antispasmodic, aphrodisiac, astringent, carminative, insecticidal, and parasiticide (CitationMajeed & Mahmood, 1988). In addition, its antimicrobial activity has also attracted great attention from many researchers (CitationChang et al., 2001; CitationAlzoreky & Nakahara, 2003). Moreover, C. zeylanicum oil has inhibitory effects against meat spoilage organisms (CitationOuattara et al., 1997), pathogenic bacteria (CitationPrabuseenivasan et al., 2006), fungi (CitationMishra et al., 2000), and viruses (CitationPacheco et al., 1993).
The main constituent of C. zeylanicum bark oils is cinnamaldehyde (more precisely trans-cinnamaldehyde or 3-phenyl-2-propenal), which is recognized as a safe food additive and widely used as a flavoring agent. Cinnamaldehyde, C9H8O (), an aromatic aldehyde, has been proved to have antitumor (CitationKa et al., 2003) and strong antifungal activities against a wide variety of wood decay fungi (CitationWang et al., 2005).
In spite of all the information available on C. zeylanicum bark oil, we were not able to find an extensive isolation study of cinnamaldehyde. Thus, we report herein the isolation, identification, and purification of cinnamaldehyde from C. zeylanicum bark oil using different spectral techniques, and its antibacterial activity against some selected pathogenic bacteria.
Materials and methods
Chemicals
CH2Cl2, benzene, MeOH, and DMSO were obtained from BDH Analar (England). Resazurin indicator tablet was obtained from Thompson and Capper Ltd (England). Tollen’s reagent and trans-cinnamaldehyde (standard) 99% purity (FW 132.16, b.p. 248°C, D 1.048) were obtained from Aldrich Chemical Company (Germany).
Plant material
C. zeylanicum bark was purchased from a local market in Mosul city, Nineveh Province, Iraq. The plant bark was identified by a botanical taxonomist at the College of Agriculture and Forestry, University of Mosul.
Essential oil extraction and isolation of major constituent
Fresh bark (1.5 kg) was subjected to steam distillation in a Clevenger-type apparatus for 8 h. The distillate (water plus essential oil) was transferred into a 125-mL separating funnel. CH2Cl2 (8 mL) was added to the separating funnel, which was then capped tightly and shaken vigorously with occasional venting. Layers were separated, and the lower CH2Cl2 layer was transferred to a clean beaker. The extraction process was repeated with two more 8-mL portions of CH2Cl2 and all of the fractions were collected. The combined CH2Cl2 layers were washed with 10–20 mL distilled water and separated into a dry container. The organic layer was dried with three or four small spatulas of anhydrous Na2SO4 and allowed to set for 10–15 min with occasional swirling, then rinsed with CH2Cl2 which was finally evaporated with gentle heating.
Characterization of pure cinnamaldehyde
Chemical detection
Cinnamaldehyde was detected according to Tollen’s test (CitationCheronis & Entrikin, 1963). Cinnamaldehyde (30 mg) was added to a test tube containing 2 mL of Tollen’s reagent. The tube was then placed in a water bath set at 35°C for 5 min. A silver mirror resulted around the test tube, which confirms that the reactant under test is an aldehyde.
TLC analysis
The isolated compound (cinnamaldehyde) from C. zeylanicum bark oil was dissolved in appropriate solvent, applied to silica gel plates, Merck (Germany) 20 cm × 20 cm, 0.25 mm in thickness, and developed using a solvent system comprising benzene–MeOH (8:2). The separated cinnamaldehyde zone was visualized under a UV lamp. Standard cinnamaldehyde served as positive control.
FTIR studies
The IR spectrum of cinnamaldehyde was recorded in the College of Education, Department of Chemistry, University of Mosul, using a computerized Tensor 27 FTIR spectrometer (Bruker Co., Germany) in the range of 400–4000 cm−1 by the KBr pellet technique.
HPLC analysis
HPLC analysis was performed in the College of Science, University of Mosul, using a Shimadzo LC 2010 HPLC system (Japan), equipped with a Shimadzo LC 2010 UV–VIS detector with a thermostatted flow cell and two selectable wavelengths of 190–370 nm or 371–600 nm. The detector signal was recorded on a Shimadzo LC 2010 integrator. The column used was a C18 block heating-type Shim-pack VP-ODS (4.6 mm i.d.×150 mm long) with a particle size of 5 μm. Cinnamaldehyde was separated with a mobile phase of water–MeOH (40:60 v/v) at a flow rate of 1.0 mL/min, column temperature 35°C. Injection volume was 20 μl and detection was carried out at 260 nm (CitationSadek, 2002).
Bacterial strains
All microorganisms were obtained from the Department of Biology, College of Education, University of Mosul. Four strains of Gram-negative bacteria (Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae, Pseudomonas aeruginosa) and two strains of Gram-positive bacteria (Staphylococcus aureus, Bacillus cereus) were used as test bacteria. The cultures of bacteria were maintained in their appropriate agar slants at 4°C throughout the study and used as stock cultures.
Antibacterial activity
Disk diffusion assay
The microorganisms were grown overnight at 37°C in 10 mL of nutrient broth. The cultures were adjusted with sterile saline solution to obtain turbidity comparable to that of McFarland no. 0.5 standard (1.0 × 108 cfu/mL) (CitationHernández et al., 2007). A modified agar diffusion method (CitationMothana & Lindequist, 2005) was used to determine antibacterial activity with slight modifications. Nutrient agar was inoculated with microbial cell suspension (200 μL in 20 mL of medium) and poured into sterile Petri dishes. Sterile filter paper disks 6 mm in diameter were impregnated with 20 μL of cinnamaldehyde in different concentrations (1:1, 1:5, 1:10 and 1:20, initially prepared by dissolving in DMSO and sterilized by filtration through 0.45 μm Millipore filters) and placed on the inoculated agar surface. A standard 6 mm disk containing streptomycin (25 μg/ disk) Bioanalyse (Germany) was used as positive control. The plates were incubated overnight at 37°C and the diameter of any resulting zones of growth inhibition was measured (mm). Each experiment was tested in triplicate.
Micro-well dilution assay
Preparation of resazurin solution.
The resazurin solution was prepared by dissolving a 270 mg tablet in 40 mL of sterile distilled water. A vortex mixer was used to ensure that it was a well-dissolved and homogeneous solution.
Preparation of the plates.
The minimal inhibitory concentration (MIC) values of cinnamaldehyde were determined based on a micro-well dilution method as previously described by CitationSarker et al. (2007), with modifications. A stock solution of cinnamaldehyde was prepared in 10% DMSO and then serial two-fold dilutions were made in a concentration range from 7.8 to 1000 μg/mL. The 96-well plates were prepared by dispensing, into each well, 95 μL of nutrient broth, 100 μL of cinnamaldehyde and 5 μL of the inoculants. The inoculums of microorganisms were prepared using 24 h cultures and suspensions were adjusted to McFarland standard turbidity. The final volume in each well was 200 μL. A positive control containing a broad-spectrum antibiotic (streptomycin in a serial dilution of 1000–7.8 μg/mL) was included on each microplate. As an indicator of bacterial growth, 10 μL of resazurin solution was added to the wells. Plates were wrapped loosely with cling film to ensure that bacteria did not become dehydrated and prepared in triplicate, and then they were placed in an incubator at 37°C for 24 h. Color change was then assessed visually. Any color change from purple to pink or colorless was recorded as positive. The lowest concentration at which color change occurred was taken as the MIC value. The average of three values was calculated as the MIC for the test material.
Results
The present study was conducted to isolate the main bioactive compound from C. zeylanicum bark oil. Cinnamaldehyde was isolated from the extracted essential oil, and then detected on TLC plates in comparison with standard cinnamaldehyde. Both isolated and standard cinnamaldehyde had the same retention factor (Rf) value of 0.69; the Rf data corroborate with CitationSilverman (1983).
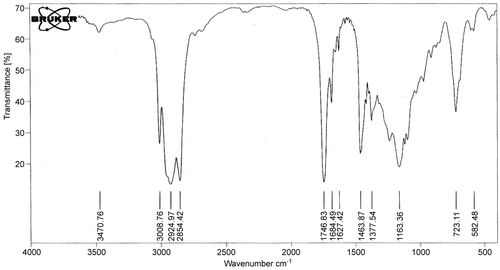
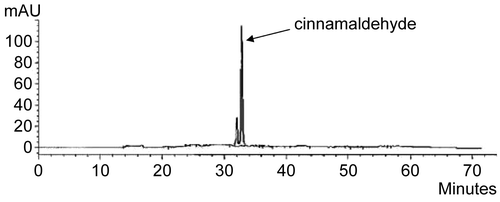
The FTIR spectrum confirmed the material isolated from C. zeylanicum bark oil as trans-cinnamaldehyde (). Significant peaks were found at: 1648–1746 cm−1 (s) corresponding to the carbonyl group C=O; 1463–1627 cm−1 (v) ascribed to the C=C bond; 3008 cm−1 (m) ascribed to the aromatic C–H bond; 2924 cm−1 (s) corresponding to the =C–H bond; 2854 cm−1 (s) ascribed to the C–H bond of the carbonyl group; and 116.63 cm−1 (v) corresponding to the aromatic C–H bond (CitationSilverstein et al., 1981), all of which confirm the purity of the isolated material. Moreover, cinnamaldehyde was characterized using HPLC () and identified by comparing its retention time (tR) and UV spectrum with that of the standard compound.
After identification, cinnamaldehyde was investigated for its antibacterial activity against six bacterial species. The initial screening of antibacterial activity of cinnamaldehyde was assayed in vitro by the agar diffusion method using four concentrations (1:1, 1:5, 1:10 and 1:20). All cinnamaldehyde concentrations were active against all tested bacteria (). The highest inhibitory effect was observed against B. cereus (zone of inhibition: 25.3 mm) using the concentration 1:1, while the weakest activity was demonstrated against P. aeruginosa (zone of inhibition: 14.4 mm) using the concentration 1:20.
Table 1. Antibacterial activity of cinnamaldehyde isolated from Cinnamomum zeylanicum bark oil.
In view of the results obtained by the disk diffusion method, the MIC of cinnamaldehyde isolated from C. zeylanicum bark oil was determined by broth microdilution assay (). B. cereus was found to be the most sensitive pathogen to cinnamaldehyde with MIC value of 31.2 μg/mL, while S. aureus, E. coli, and K. pneumonia ranked next (MIC 62.5 μg/mL) followed by P. mirabilis and P. aeruginosa with MIC of 125.0 μg/mL. The standard drug streptomycin was active against all reference bacteria (zone of inhibition range: 15.3–22.3 mm; MIC range: 7.8–15.6 μg/mL).
Table 2. Minimum inhibitory concentration (MIC) of cinnamaldehyde isolated from Cinnamomum zeylanicum bark oil.
Discussion
Essential oils are aromatic oily liquids obtained from plant materials. They can be obtained by expression, fermentation, or extraction, but steam distillation is the most commonly used method (CitationBurt, 2004). Essential oils are complex mixes comprising many of single compounds. Chemically, they are derived from terpenes and their oxygenated compounds. Each of these constituents contributes to the beneficial or adverse effects (CitationPrabuseenivasan et al., 2006). Some essential oils have antibacterial (CitationWannissorn et al., 2005), antifungal (CitationNakamura et al., 2004), antiviral (CitationBishop, 1995), antitoxigenic (CitationJuglal et al., 2002), and antiprotozoal (CitationHoletz et al., 2003) properties.
In the present study, different physical methods were employed to characterize cinnamaldehyde. Among them, the IR spectrum and HPLC indicated the absolute purity of the isolated material. The retention time and UV spectrum of the isolated material on HPLC was completely identical to that of standard cinnamaldehyde. HPLC is the most widely used qualitative and quantitative determination and separation method. The method is popular because it is non-destructive and may be applied to thermally labile compounds (unlike GC); it is also a very sensitive technique since it incorporates a wide choice of detection methods.
Cinnamaldehyde is the major constituent of cinnamon essential oils. It occurs naturally in the bark and leaves of cinnamon trees of the genus Cinnamomum, and is believed to have many medicinal properties. In vitro studies in the present work showed that cinnamaldehyde inhibited the growth of all tested bacteria. The zones of inhibition ranged from 14.4 to 25.3 mm in diameter using the disk diffusion method. Furthermore, MIC values ranged from 31.2 to 125.0 μg/mL, and the most promising result was observed against B. cereus. The antimicrobial activity of many essential oils has been previously reviewed and classified as strong, medium, or weak (CitationMatan et al., 2006). Several studies have shown that cinnamon, clove, and rosemary oils had strong and consistent inhibitory effects against various pathogens (CitationAureli et al., 1992; CitationMatan et al., 2006). Several GC-MS studies have revealed that cinnamaldehyde is the major constituent, and the predominant active compound, found in cinnamon oil (CitationBaratta et al., 1998; CitationSimic et al., 2004).
Although the antimicrobial properties of essential oils and their components have been reviewed in the past (CitationShelef, 1983; CitationNychas, 1995), the mechanism of action has not been studied in great detail (CitationLambert et al., 2001). Considering the large number of different groups of chemical compounds present in essential oils, it is most likely that their antibacterial activity is not attributable to one specific mechanism but that there are several targets in the cell (CitationCarson et al., 2002). It is thought that the carbonyl group of cinnamaldehyde binds to proteins and prevents the action of amino acid decarboxylases in Enterobacter aerogenes (CitationWendakoon & Sakaguchi, 1995). Moreover, an important characteristic of essential oils and their components is their hydrophobicity, which enables them to partition the lipids of the bacterial cell membrane and mitochondria, disturbing the cell structures and rendering them more permeable (CitationSikkema et al., 1994). Extensive leakage from bacterial cells or the exit of critical molecules and ions will lead to death (CitationDenyer & Hugo, 1991).
In view of the results obtained using both disk diffusion and micro-well dilution assays, cinnamaldehyde was found active to be against both Gram-positive and Gram-negative bacteria. Notwithstanding, that Gram-negative organisms were slightly less susceptible to the action of cinnamaldehyde since they possess an outer membrane surrounding the cell wall (CitationRatledge & Wilkinson, 1988) which restricts the diffusion of hydrophobic compounds through its lipopolysaccharide covering (CitationVaara, 1992). In addition, the weakest activity was observed against P. aeruginosa using both antibacterial methods. Several studies have reported that the Gram-negative bacteria Pseudomonas, and in particular P. aeruginosa, appear to be least sensitive to the action of essential oils (CitationDorman & Deans, 2000; CitationPintore et al., 2002; CitationWilkinson et al., 2003).
It can be concluded that cinnamaldehyde isolated from C. zeylanicum bark essential oils had excellent antibacterial activities against six different bacterial species and that cinnamaldehyde is very much responsible for the antibacterial activity of the essential oil of this very important medicinal plant. Additional in vivo studies and clinical trials would be needed to justify and further evaluate the potential of this compound as an antibacterial agent in topical or oral applications.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alzoreky NS, Nakahara K (2003), Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol 80, 223–230.
- Aureli P, Costantini A, Zolea S (1992), Antibacterial activity of some plant essential oils against Listeria monocytogences. J Food Prot 55, 344–348.
- Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruberto G (1998), Antimicrobial and antioxidant properties of some commercial essential oils. Flav Fragr J 13, 235–244.
- Bishop CD (1995), Antiviral activity of the essential oil of Melaleuca alternifolia (Maiden & Betche) Cheel (tea tree) against tobacco mosaic virus. J Essent Oil Res 7, 641–644.
- Burt S (2004), Essential oils: their antibacterial properties and potential applications in foods – a review. Int J Food Microbiol 94, 223–253.
- Carson CF, Mee BJ, Riley TV (2002), Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother 46, 1914–1920.
- Chang ST, Chen PF, Chang SC (2001), Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol 77, 123–127.
- Cheronis ND, Entrikin JB (1963), Identification of Organic Compounds. New York, Interscience (Wiley), pp. 208–209.
- Denyer SP, Hugo WB (1991), Biocide-induced damage to the bacterial cytoplasmic membrane. In, Denyer SP, Hugo WB eds. Mechanisms of Action of Chemical Biocides. The Society for Applied Bacteriology, Technical Series No. 27. Oxford, Oxford Blackwell Scientific Publication, pp. 171–188.
- Dorman HJ, Deans SG (2000), Antimicrobial agents from plants: activity of plant volatile oils. J Appl Microbiol 88, 308–316.
- Duarte MC, Figueira GM, Sartoratto A, Rehder VL, Delarmelina C (2005), Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol 97, 305–311.
- Hernández T, Canales M, Avila JG, Duran A, Caballero J, Romo de Vivar A, Lira R (2003), Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitán de las Salinas, Puebla (México). J Ethnopharmacol 88, 181–188.
- Hernández T, Canales M, Teran B, Avila O, Duran A, Garcia A, Hernandez H, Angeles-Lopez O, Fernandez-Araiza M, Avila G (2007), Antimicrobial activity of the essential oil and extracts of Cordia curassavica (Boraginaceae). J Ethnopharmacol 111, 137–141.
- Holetz FB, Ueda-Nakamura T, Dias FilhoBP, Cortez DA, Morgado-Díaz JA, Nakamura CV (2003), Effect of essential oil of Ocimum gratissimum on the trypanosomatid Herpetomonas samuelpessoai. Acta Protozool 42, 269–276.
- Jayaprakasha GK, Jagan MohanRaoL, Sakariah KK (2003), Volatile constituents from Cinnamomum zeylanicum fruit stalks and their antioxidant activities. J Agric Food Chem 51, 4344–4348.
- Juglal S, Govinden R, Odhav B (2002), Spice oils for the control of co-occurring mycotoxin-producing fungi. J Food Prot 65, 683–687.
- Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, Lee KT (2003), Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett 196, 143–152.
- Lambert RJ, Skandamis PN, Coote P, Nychas G (2001), A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91, 453–462.
- Majeed SH, Mahmood MJ (1988), Herbs and Medicinal Plants in Iraq Between Traditional Medicine and Scientific Research, 1st ed. Baghdad, Dar Al-Thaowra Publishing, pp. 75–76 (in Arabic).
- Matan N, Rimkeeree H, Mawson AJ, Chompreeda P, Haruthaithanasan V, Parker M (2006), Antimicrobial activity of cinnamon and clove oils under modified atmosphere conditions. Int J Food Microbiol 107, 180–185.
- Mishra N, Upma K, Shukla D (2000), Antifungal activity of essential oil of Cinnamomum zeylanicum. J Essent Oil Res 3, 97–110.
- Mothana RA, Lindequist U (2005), Antimicrobial activity of some medicinal plants of the island Soqotra. J Ethnopharmacol 96, 177–181.
- Nakamura CV, Ishida K, Faccin LC, Dias FilhoBP, Cortez DA, Rozental S, De SouzaW, Ueda-Nakamura T (2004), In vitro activity of essential oil from Ocimum gratissimum L. against four Candida species. Res Microbiol 155, 579–586.
- Nychas GJ (1995), Natural antimicrobials from plants. In, Gould GW ed . New Methods of Food Preservation. London, Blackie Academic and Professional, pp. 58–89.
- Ouattara B, Simard RE, Holley RA, Piette GJ, Begin A (1997), Antibacterial activity of selected fatty acids and essential oils against six meat spoilage organisms. Int J Food Microbiol 37, 155–162.
- Pacheco P, Sierra J, Schmeda-Hirschmann G, Potter CW, Jones BM, Moshref M (1993), Antiviral activities of Chilean medicinal plant extracts. Phytother Res 7, 415–418.
- Pintore G, Usai M, Bradesi P, Juliano C, Boatto G, Tomi F, Chessa M, Cerri R, Casanova J (2002), Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Frag J 17, 15–19.
- Prabuseenivasan S, Jayakumar M, Ignacimuthu S (2006), In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med 6, 39–46.
- Ratledge C, Wilkinson SG (1988), An overview of microbial lipids. In, Ratledge C, Wilkinson SG eds. Microbial Lipids. London, Academic Press, pp. 3–22.
- Sadek PC (2002), The HPLC Solvent Guide, 2nd ed. New York, John Wiley & Sons, Inc., pp. 643–655.
- Sarker SA, Nahar L, Kumarasamy Y (2007), Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42, 321–324.
- Shelef LA (1983), Antimicrobial effects of spices. J Food Safety 6, 29–44.
- Sikkema J, De Bont JA, Poolman B (1994), Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269, 8022–8028.
- Silverman RB (1983), Mechanism of inactivation of monoamine oxidase by trans-2-phenylcyclopropylamine and the structure of the enzyme–inactivator adduct. J Biol Chem 208, 14766–14769.
- Silverstein RM, Bassler GC, Morrill TC (1981), Spectrometric Identification of Organic Compounds, 4th ed. New York, John Wiley & Sons, Inc., pp. 81–92.
- Simic A, Sokovic MD, Ristic M, Grujic-Jovanovic S, Vukojevic J, Marin PD (2004), The chemical composition of some Lauraceae essential oils and their antifungal activities. Phytother Res 18, 713–717.
- Vaara M (1992), Agents that increase the permeability of the outer membrane. Microbiol Rev 56, 395–411.
- Wang SY, Chen PF, Chang ST (2005), Antifungal activities of essential oils and their constituents from indigenous cinnamon (Cinnamomum osmophloeum) leaves against wood decay fungi. Bioresour Technol 96, 813–818.
- Wannissorn B, Jarikasen S, Siriwangchai T, Thubthimthed S (2005), Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 76, 233–236.
- Wendakoon CN, Sakaguchi M (1995), Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Prot 58, 280–283.
- Wilkinson JM, Hipwell M, Ryan T, Cavanagh HM (2003), Bioactivity of Backhousia citriodora: Antibacterial and antifungal activity. J Agric Food Chem 51, 76–81.