Abstract
The rise of infection caused by ‘superbugs’ is alarming and one of the most problematic resistant bacteria is methicillin-resistant Staphylococcus aureus (MRSA). This bacterium can cause a range of ailments like pneumonia, mastitis, meningitis, urinary tract infection, and post operational infection. Ten medicinal plants were investigated for their efficacy against drug-sensitive and drug-resistant strains of S. aureus. Ethanol extracts of Melianthus comosus Vahl (Melianthaceae), Melianthus major L (Melianthaceae), Dodonaea viscosa Jacq. var. angustifolia (L.f.) Benth (Sapindaceae) and Withania somnifera L. Dunal (Zygophyllaceae) were found to have good inhibitory activity against both drug-sensitive and drug-resistant strains of S. aureus. Minimum inhibitory concentrations of these plants ranged from 0.391 to 1.56 mg/ml. Ethanol extracts of all these plants were further tested for cytotoxicity on Vero cells using the XTT method. M. major exhibited a 50% inhibitory concentration (IC50) of 52.76 μg/ml and was, therefore, selected for the identification of bioactive principles. Two flavonoid compounds namely, quercetin 3-O-β-galactoside-6-gallate and kaempferol 3-O-α-arabinopyranoside, were isolated from the leaves using column chromatography. These compounds were isolated for the first time from this plant. These flavonoids did not show antibacterial activity against methicillin-sensitive strain of Staphylococcus aureus at the highest concentration (500 μg/ml) tested. The antibacterial activity of ethanol extract of M. major observed in this study could be either due to the synergistic activity of compounds present in the extract and/or due to compounds which have not been purified in this study. Good antibacterial activity of three plant extracts, namely, Melianthus comosus, Melianthus major, and Dodonaea viscose, as observed especially against MRSA, supports the use of extracts by South Africans for infections caused by S. aureus to some extent.
Introduction
Naturally occurring substances of plant, animal, and mineral origin have provided a continuing source of medicines since the earliest times known to man, but it is the plant kingdom, in particular, which has proved to be of most use for treating many of our ailments. During the course of history, experimentation has succeeded in distinguishing those plants which have beneficial effects from those which are toxic or merely non-effective. Through trial and error, human beings have discovered ways of relieving pain and sickness, and of living in harmony with nature. This process has gradually evolved throughout the world over a period of thousands of years and it is estimated that about 20,000 plant species are used medicinally (CitationO’Neill, 1993). Medicinal plants play an important part for the primary health system in South Africa and it is estimated that 80% of the black population currently consult traditional healers for treatment (CitationJäger et al., 1996; CitationO’Neill, 1993).
The rise of the resistant strain of Staphylococcus aureus in the world is of concern. The resistance is caused by the bacteria acquiring genes that produce the enzyme β-lactamase that inactivates antibiotics (CitationChang et al., 2003). In general, about 60% of the cases of staphylococcal infection reported in hospitals are caused by methicillin-resistant Staphylococcus aureus (MRSA). The antibiotics needed to treat the resistant strain usually have very serious side effects. Vancomycin is used for infections caused by MRSA. The side effects experienced with this drug are rashes at the site of injection and sometimes it can lead to even deafness, when the level of this drug in the blood becomes too high. This prompts researchers to discover novel drugs with no side effects that could be used to treat infections caused by the resistant bacteria.
The major objective of this study was to investigate the activity of ten plant extracts against methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA bacteria. All these plants are being used by South African traditional healers to treat symptoms that are related to infections caused by S. aureus. Cytotoxic tests were conducted to determine the potential of these extracts for future use as therapeutic agents. An attempt was made to isolate and purify compounds from the extracts exhibiting minimum inhibitory concentration (MIC) < 1.0 mg/ml against MRSA.
Materials and methods
Plant material
Ten plants, namely, Albizia harveyii E. Fourn. (Leguminosae), Boophane disticha L.f. Herb (Amaryllidaceae), Dodonaea viscosa Jacq. var. angustifolia L.f. Benth. (Sapindaceae), Euphorbia damarana L.C. Leach (Euphorbiaceae), Melianthus comosus Vahl (Melianthaceae), Melianthus major L. (Melianthaceae), Piper capense L.f. var. capense and Withania somnifera L. Dunal (Zygophyllaceae) were obtained during January and February 2006 from Pretoria (South Africa). Another two plants, Drimia altissima L.f. Ker Gawl (Hyancinthaceae) and Plantago longissima Decne (Plantaginaceae), were collected from farms in Northern and North-east KwaZulu-Natal (South Africa) in January 2006. Different parts of the plants such as leaves, bark, roots, etc., as used traditionally for the treatment of Staphylococcal-infections were collected. The plants were identified by the taxonomist (Prof. B. Van Wyk) at the HGWJ Schweickerdt herbarium of the University of Pretoria. Voucher specimens are preserved in the herbarium of the University of Pretoria ().
Table 1. Activity of the ethanol extracts of selected plants against methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus.
Extract preparation
Dried plant parts of all ten plants (20 g) were extracted with ethanol. The extract was filtered and concentrated to dryness at reduced pressure. The resultant residue was later dissolved in 10% dimethyl sulphoxide (DMSO) to a concentration of 100 mg/ml.
Subculturing of bacteria
Two bacterial species, methicillin-sensitive Staphylo- coccus aureus (MSSA) (ATCC 12600) and MRSA (GTS 656167) were obtained from the Department of Microbiology and Pathology, at the University of Pretoria. Each organism was maintained on nutrient agar slant, and was recovered for testing by growing them in nutrient broth (Merck) for 24 h at 37˚C.
Microtitre bioassay
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of all plant extracts were determined using Micro-dilution technique using 96-well microtitre plates, as described by CitationEloff (1998) against MSSA and MRSA strains of S. aureus. The extracts were first dissolved in 10% DMSO and then added to the nutrient broth (NB) to obtain final concentration of 25 mg/ml in the first well. This was serially diluted two-fold to obtain a concentration range of 25-0.01 mg/ml for extracts. The antibiotic gentamicin sulphate (BioWhittaker™) at concentrations ranging from 4.0 to 0.002 μg/ml served as positive drug control (CitationMcGaw & Eloff, 2005). The final concentration of DMSO in the wells was ≤ 2.5% which did not have any inhibitory effect on the test organism. Bacterial inoculum (100 μl) (2.45 × 106 CFU/ml) of MSSA and MRSA was added to the wells thereafter, the plates were incubated at 37°C for 24 h. Microbial growth was indicated by adding 40 μl of (0.2 mg/ml) of p-iodonitrotetrazoilium violet (INT) (Sigma-Aldrich, South Africa) to microtitre wells and were re-incubated at 37°C for 1 h. MIC was defined as the lowest concentration of extract that inhibited the color change of INT.
The minimum bactericidal concentration (MBC) for each strain was determined by adding 50 μl from microtitre wells which did not show any bacterial growth after incubation during MIC bioassays to 150 μl of fresh nutrient broth. Plates were re-incubated for another 24 h. MBC endpoints were read as the lowest dilution of extract with no growth after overnight incubation at 37˚C (CitationReimer et al., 1981).
Cytotoxicity test
Four extracts which showed good activity against both the strains of S. aureus were further tested for cytotoxicity on Vero cells. Vero cells, from African green monkey kidney cells (ATCC CCL-81), were maintained in culture flasks in complete MEM (Minimum Essential Medium Eagle). Subculturing was done every 2-3 days after it had formed a confluent monolayer. When the cells were subcultured the cells that attached to the culture flask were trypsinized (0.25% trypsin containing 0.01% EDTA) for 10 min at 37˚C in an atmosphere with 5% CO2 and 95% humidity. The addition of complete medium to the cell suspension stopped the trypsinizing. About 1 × 105 of the viable cells were then re-suspended in complete medium (CitationZheng et al., 2001).
Cytotoxicity was measured by the XTT method using the Cell Proliferation Kit II (Roche Diagnostics GmbH). The Vero cells was seeded at 1x105 onto a microtiter plate and incubated for 24 h to allow the cells to attach to the bottom of the plate. A dilution series was made of the extracts and the various concentrations ranging from 200 μg/ml to 3.1 μg/ml were added to the microtiter plate and incubated for 48 h. Zelaralenone served as positive drug control for this assay. The XTT reagent at a final concentration of 0.3 mg/ml was added to the wells which were reincubated for 1-2 h. After incubation the absorbance of the color complex was spectrophotometrically quantified using an ELISA plate reader, which measured the optical density at 490 nm with a reference wavelength of 690 nm (CitationZheng et al., 2001). The IC50 is defined as the reciprocal deduction resulting in 50% inhibition of Vero cells (CitationWoldemichael et al., 2004). The statistical programme (Garph Pad Prism 4) was used to analyze the IC50 values.
Isolation and purification
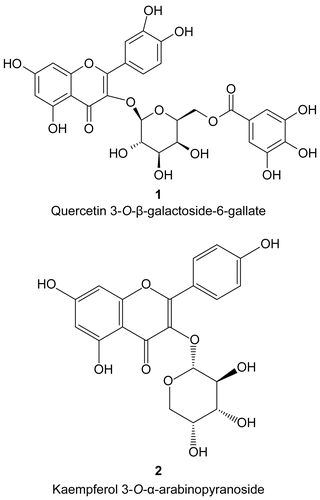
Ethanol extracts of Melianthus comosus, Melianthus major, Dodonaea viscosa var. angustifolia, and Withania somnifera were found to have good inhibitory activity against both drug-sensitive and drug-resistant strains of S. aureus. Dodonaea angustifolia and Melianthus comosus showed better activity than most of the plants tested, but due to unavailability of plant material it was decided to isolate compounds from the next best plant (Melianthus major). Air-dried leaves of M. major (1.315 kg) was extracted with ethanol (2 × 2 L) for 48 h at room temperature (± 25oC). The ethanol crude extract was filtered and evaporated under reduced pressure. The total concentrated extract (98 g) was subjected to silica gel column chromatography (CC) using hexane/ethyl acetate (EtOAc) mixtures of increasing polarity (0 to 100%) followed by 100% methanol. In total, 12 fractions (500 ml) were collected. Similar fractions, according to TLC profile, were combined into three fractions. The three fractions were assayed using thin-layer chromatography (TLC) bioautography against S. aureus. Fraction 2 showed prominent zones of inhibition and, therefore, was further subjected to another silica column using dichloromethane (DCM) and MeOH mixtures of increasing polarity. Seven fractions were obtained. The fourth subfraction of fraction 2 was rechromatographed on Sephadex LH-20 column 100% ethanol (EtOH) as eluents to give compound 1 (yield 0.003%). The sixth subfraction of fraction 2 was purified on a preparative TLC plate (silica gel 60 F254) using 3% MeOH in CHCl3 which gave compound 2 (yield 0.0006%). The structural elucidation of isolated compounds were done by their physical (m.p., [α]D and spectroscopic (1H-NMR, and 13C-NMR) data.
Results and discussion
Inhibitory activity of plant extracts and isolated compounds
The ethanol extracts of all plants showed moderate activity on the drug sensitive S. aureus bacteria. The extracts of M. major and M. comosus inhibited the growth of S. aureus at 0.558 and 0.500 mg/ml, respectively (). M. major and M. comosus both had significant bactericidal effect at 1.177 and 2.00 mg/ml, respectively. The results from this study correlated with that of CitationMcGaw and Eloff (2005) to some extent. It has been reported that Melianthus major and M. comosus had MIC values of 0.780 mg/ml on the sensitive strain of S. aureus. Dodonaea angustifolia and Withania somnifera also showed good inhibition at 0.500 mg/ml when tested on the sensitive strain of S. aureus. The positive control, ‘Gentamicin’ had a much lower MIC value (2.00 μg/ml) than that of the extracts and isolated compounds when tested against Staphylococcus aureus. The MIC observed for M. comosus was the lowest, i.e., 0.391 mg/ml against the drug-resistant strain of S. aureus (). Previous studies have shown inhibition of the MRSA strain to be at 1.0 mg/ml (Curcuma longa) and higher (CitationKim et al., 2005).
Cytotoxicity
The IC50 values of plant extracts were found to be at 52.76 μg/ml (M. major), 51.41 μg/ml (M. comosus), 42.11 μg/ml (D. angustifolia) and 20.18 μg/ml (W. somnifera) (). The W. somnifera was previously tested for its cytotoxicity; these results were obtained using human ECV 304 cells (CitationAl-Fatimi et al., 2005). The IC50 values (1.1 μg/ml) obtained by CitationAl-Fatimi et al. (2005) were much lower than those obtained in this study (20.18 μg/ml).
Table 2. Fifty percent inhibitory concentration of ethanol extracts of selected plantand compounds isolated from M. major against Vero cells.
Identification of isolated compounds
Two flavonoid compounds, obtained by column chromatographic purification of the ethanol extracts of M. major, were quercetin 3-O-β-galactoside-6-gallate and kaempferol 3-O-α-arabinopyranoside (). Compounds were identified by analyzing their 1H-NMR and 13C-NMR and 2D spectral data (COSY, HMQC, HMBC, and NOESY). The recorded spectra were compared with published data (CitationPhol et al., 1975; CitationKim et al., 2005). Both compounds are being reported for the first time from Melianthus major.
The activity of the two flavonoid compounds was tested using the microdilution method of CitationEloff (1998). The results obtained did not indicate any significant inhibition of the bacteria at the highest concentration tested (500 μg/ml) for both compounds. The cytotoxic effect of the ethanol crude extract and two pure compounds isolated from M. major in this study is shown in . It appears that the pure compounds, compounds 1 and 2, have moderate IC50 values (64.27 and 160.7 μg/ml, respectively), thus, suggesting lower toxicity as compared to the crude extract (IC50 52.74 μg/ml). However, from the results of this study, it appears that compound 2 is less cytotoxic to Vero cells as compared to compound 1 and the crude extract. The antibacterial activity of ethanol extract of M. major observed in this study could be either due to the synergistic activity of compounds present in the extract and/or due to compounds which have not been purified in this study. Good antibacterial activity of three plant extracts, namely, Melianthus comosus, Melianthus major and Dodonaea viscosa, as observed specially against MRSA of S. aureus supports the use of extracts by South Africans for infections caused by S. aureus to some extent.
Acknowledgements
The authors are grateful to the Pathology Department, University of Pretoria for the assistance in testing on the MRSA and to Mr. P. V. Maltiz, the traditional healer for sharing his knowledge with them.
Declaration of interest: The authors report no conflicts of interest.
References
- Al-Fatimi M, Friedrich U, Jenett-Siems K (2005), Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia 76, 355–358.
- Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK (2003), Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. New Engl J Med 348, 1342–1347.
- Eloff JN (1998), A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64, 711–713.
- Jäger AK, Hutchings A, Van Staden J (1996), Screening of Zulu medicinal plants for prostaglandin-synthesis inhibitors. J Ethnopharmacol 52, 95–100.
- Kim KJ, Yu HH, Cha JD, Seo SJ, Choi NY, You YO (2005), Antibacterial activity of Curcuma longa L. against methicillin-resistant Staphylococcus aureus. Phytother Res 19, 599–604.
- Kim SJ, Cho JY, Wee JH, Jang MY, Kim C, Rim YS, Shin SC, Ma SJ, Moon JH, Park KH (2005), Isolation and characterization of antioxidative compounds from the aerial parts of Angelica keiskei. Food Sci Biotechnol 14 (1), 58–63.
- McGaw LJ, Eloff JN (2005), Screening of 16 poisonous plants for antibacterial, anthelmintic and cytotoxic activity in vitro. S Afr J Bot 71, 302–306.
- O’Neill MJ (1993), The renaissance of plant research in the pharmaceutical industry. In, A.D. Kinghorn and M.F. Balandrin. Human Medicinal Agents in Plants. American Chemical Society, Washington, pp. 48–55.
- Phol R, Janistyn B, Nahrstedt A (1975), Flavonol glycosides from Euphorbia helioscopia, E. stricta, E. verrucosa, and E. dulcis. Planta Med 27, 301–303.
- Reimer GL, Stratton CW, Reller LB (1981), Minimum inhibitory and bactericidal concentration of 44 antimicrobial agents against three standard control strains in broth with and without human serum. Antimicrob Agents Chemother 19, 1050–1055.
- Woldemichael GM, Gutierrez-Lugo MT, Franzblau SG, Wang Y, Saurez E, Timmermann BN (2004), Mycobacterium tuberculosis growth inhibition by constituents of Sapium haematospermum. J Nat Prod 67, 598–603.
- Zheng YT, Chan WL, Chan P, Huang H, Tam SC (2001), Enhancement of the anti-herpetic effect of trichosanthin by acyclovir and interferon. FEBS Letters 496, 139–142.