Abstract
Rheum ribes Linn (Polygonaceae) root is used traditionally to treat diabetes, hemorrhoids, ulcers, and diarrhea. Here, the hypoglycemic effect of R. ribes root extract in healthy mice was investigated. Fasted mice were given a single dose of 50 mg/kg of three extracts of different polarity from R. ribes by gastric feeding and the blood glucose was measured 0, 1, 2, 4, and 24 h later. The aqueous extract showed a significant hypoglycemic effect. In vitro, the aqueous extract stimulated insulin release from INS-1E cells at both stimulatory (20 mM) and non-stimulatory (1 mM) glucose concentrations, thus suggesting a mechanism for the in vivo effect. The hypoglycemic active fraction was found to contain anthraquinone glycosides of aloe emodin, emodin, physcion, and chrysophanol derivatives.
Introduction
Rheum ribes Linn (Polygonaceae) is an Iraqi species of rhubarb which is found in Turkey, Iran, Pakistan, Afghanistan, and Russia (CitationDavis, 1967). Roots of R. ribes are used traditionally to treat diabetes, hemorrhoids, ulcers and diarrhea, and have been reported to have anthelmentic and expectorant activities (CitationTabata et al., 1994; CitationTosun & Akyuz Kizilay, 2003). A number of anthraquinone and stilbene compounds were previously separated and identified as the main chemical constituents in the roots of R. ribes (CitationMericli & Tuzlaci, 1990; CitationAlaadin et al., 2007). Some species of rhubarb were found to have hypoglycemic activity (Citationözbek et al., 2004; CitationChoi et al., 2005; CitationSuresh et al., 2004). In the present study we examined the antidiabetic effect of three types of extracts from R. ribes roots on normoglycemic mice in addition to the insulin stimulatory effect on INS-1E cells. The phytochemical profiles were evaluated to ascribe the biological activity of this plant.
Materials and methods
Plant material
R. ribes roots were collected from the mountains of Haji Omaran, Kurdistan region, Iraq during May 2006. The roots were cleaned, cut into small pieces, air dried under shade for 5–7 days and stored in bottles until used. The identity of the plant was confirmed by Assistant Professor Al-khayat AH. A voucher specimen is stored at the Department of Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Copenhagen (voucher No. Alaadin 1).
Extract preparation
R. ribes roots were extracted with 80% ethanol and the extract was evaporated to dryness in vacuo with a rotary evaporator yielding 13.8% (w/w) total extract A. Liquid partitioning of A between CHCl3:H2O yielded 0.75% (w/w) chloroform extract B and 11.2% (w/w) aqueous fraction C after freeze-drying (Hetosicc, Heto Lab Equipment, Denmark).
Experimental animals
Adult NMRI mice (male, 25–30 g body weight) were obtained from Taconic A/S (Denmark). They were housed in groups of 6 per cage at a temperature of 25° ± 1°C and relative humidity of 45% to 55% under a 12 h light/12 h dark cycle. The animals were given a commercial feed and allowed tap water ad libitum. The experiment was approved by the Danish Animal Experimentation Inspectorate.
Twenty-five mice were divided randomly into groups with 5 mice each. Animals given 0.5 ml of 20% (v/v) Tween 80 in isotonic saline served as negative controls and animals given 0.5 ml (5 mg/kg) glibenclamide (Sigma-Aldrich, Germany) as positive controls. Three other groups were given 0.5 ml (50 mg/kg) each of extracts A, B and C, respectively. Substances were prepared as suspensions of extract in 20% (v/v) Tween 80 in isotonic saline. All the tested drugs were administered intragastrically by cannula. Animals were fasted for 5 h prior to drug administration and blood samples were obtained 0, 1, 2, 4, and 24 h after administration of the test substances. Blood glucose was measured using Ascentia Elite test strips (Bayer).
In vitro insulin release assay
INS-1E were grown in RPMI 1640 (Cambrex) supplemented with 10% fetal calf serum (FCS, Gibco), 10 mM Hepes buffer, 1 mM pyruvate, and 50 μM β-mercaptoethanol in a humidified 5% CO2 atmosphere. The cells were used in passages 70-100.
The cells were seeded in 12-well plates (Costar) at 70% confluence 24 h prior to experiments. The medium was then changed to RPMI 1640 with 0.5% FCS for 24 h, then to glucose-free RPMI with 0.5% FCS for 2 h, finally to Ca5-buffer (125 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 1.2 mM MgCl2 hexahydrate, 25 mM Hepes, 0.1% BSA, pH 7.4) for 30 min. The stimulation was carried out for 30 min in the presence of either 1 or 20 mM glucose and 0-100 μg/mL of extract C. The supernatants were spun at 200 g for 5 min and frozen for later insulin measurement. Insulin was determined using a rat insulin ELISA kit (Mercodia, Sweden). The insulin concentration in samples without extract was used for normalizing the results to calculate the relative insulin release. The insulin release was induced 3.7 ± 0.9 fold by 20 mM glucose compared to medium with 1 mM glucose (P = 0.001, N = 16).
Statistical analysis
In vivo results were tested by one-way and repeated measures ANOVA and Fisher’s LSD multiple-comparison test. In vitro insulin secretion index was tested by one-sample Student’s t-test.
Identification of the constituents in extract C
Modified Borntrager’s test was used for the qualitative identification of extract C (CitationEvans, 2002). An aliquot of 5 g of fraction C was dissolved in 50 mL water, refluxed with 10 ml FeCl3 60% for 30 min, 10 mL of HCl 5% was added to the mixture which was heated for further 30 min. The resulting mixture was cooled and fractionated with chloroform (CitationHMSO, 1993) to yield 2.1% of organic extract C1 after drying by rotary evaporator.
Thin-layer chromatography (TLC) of extract (C1)
TLC evaluation of extract C1 was carried out on silica gel GF254, 0. 2 mm thickness aluminum plates, with petroleum ether:ethyl acetate:formic acid (75:25:1) as a mobile phase (CitationHMSO, 1993). Aloe emodin, emodin, physcion, and chrysophanol standard anthraquinone compounds were used as references.
Results
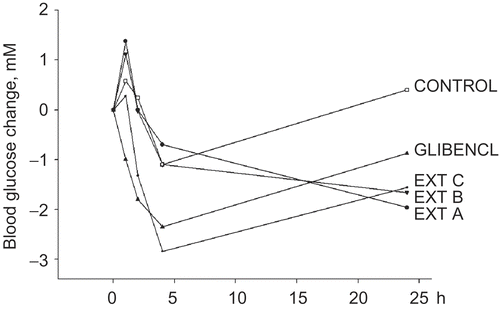
For the in vivo investigations, it was first examined if the various R. ribes extracts resulted in identical reductions in blood glucose (). As testing parameter, the maximum decrease in blood glucose for each individual mouse was used, and one-way ANOVA analysis showed that this was not the case (P = 0.01, N = 25). This was also confirmed by repeated measures ANOVA (P = 0.04, N = 25) in which all measured blood glucose values were included as changes from the value at t = 0. Fisher’s LSD multiple-comparison test subsequently clustered extract C and glibenclamide in a group which differed significantly from extract B and the control, showing that extract C contained a hypoglycemic agent while extracts A and B did not ().
Table 1. Effect of oral administration of extracts of Rheum ribes root on plasma glucose concentration in healthy mice. The values represent the mean ± SEM.
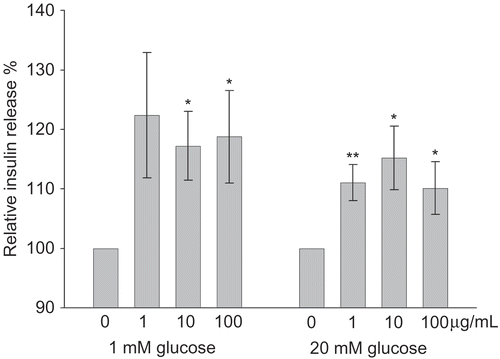
For in vitro investigations of extract C under non-stimulatory conditions (1 mM glucose), insulin release was significantly increased at 10 and 100 μg/mL by 17.2 ± 6% and 18.8 ± 8% (P = 0.012 and 0.03, N = 12). During stimulatory conditions (20 mM glucose), all tested extract concentrations significantly increased the insulin release by 11.1 ± 3%, 15.2 ± 5%, and 10.2 ± 4% (P = 0.004, P = 0.02, P = 0.04, N = 12) for 1, 10, and 100 μg/mL, respectively ().
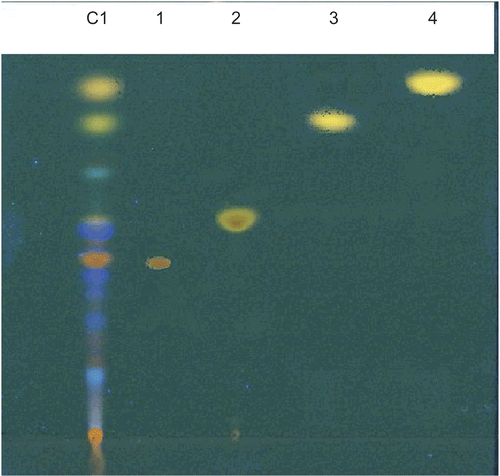
Modified Borntrager’s test resulted in red coloration of the upper aqueous layer of extract C which indicated the presence of anthraquinone derivatives in this fraction. Separation of the constituents in extract C1 by TLC revealed the presence of four types of anthraquinone derivatives, aloe emodin, emodin, physcion, and chrysophanol (), which have previously been isolated from R. ribes roots (CitationAlaadin et al., 2007).
Figure 3. TLC plate for extract C1 under UV 366 nm; 1-aloe emodin; 2-emodin; 3- physcion; 4- chrysophanol standard compound. (See colour version of this figure online at www.informapharmascience.com/phb).
Discussion
The ethnopharmacological studies on R. ribes root (Citationözbek et al., 2004) together with our data confirm that an aqueous extract of R. ribes roots shows clear hypoglycemic activity which is in accordance with the traditional use in Kurdistan for the treatment of diabetes. A recent animal study suggests that Rheum extracts are also safe to administer (CitationKaszkin-Bettag et al., 2008).
Our study showed a significant hypoglycemic effect of the aqueous extract C from R. ribes root on healthy mice, whereas the total extract A and the organic extract B did not show hypoglycemic activity. This study has also showed that the aqueous extract C from R. ribes roots is less potent than glibenclamide at the concentrations used, and that the hypoglycemic effect persisted for at least 24 h, the last time-point tested.
In vitro experiments demonstrated that the aqueous extract C could stimulate insulin release directly at both non-stimulatory and stimulatory glucose conditions (1 and 20 mM). Insulin is stored in crystals of insulin and c-peptide in large vesicles in pancreatic beta cells and released by exocytosis. Since Ca2+ regulates several steps in insulin exocytosis such as the size of vesicle pools, the fusion event, and the size of the fusion pool (CitationErnandez-Chacon & Alvarez, 1995), it is possible that the aqueous extract C could increase cytoplasmic Ca2+ by increasing the influx of extracellular Ca2+ and release of intracellular Ca2+ (CitationLi et al., 1999, Citation2001). An in vitro and in vivo study showed that desoxyrhaponticin (3,5- dihydroxy-49-methoxystilbene 3-O-β-d-glucoside) a major stilbene in rhubarb inhibits glucose uptake in the intestine and kidney, which suggests that other mechanisms could also be responsible for the antidiabetic effect (CitationLi et al., 2007).
Phytochemical investigation of the hypoglycemic fraction C revealed the presence of anthraquinone glyco sides. On hydrolysis, four types of anthraquinone aglycone derivatives were identified as aloe emodin, emodin, physcion, and chrysophanol by co-chromatography with reference compounds that give the same Rf values and color as the compounds in the extract.
In conclusion, the study demonstrated that administration of aqueous extract of R. ribes root containing anthraquinone derivatives to normal mice elicited hypoglycemia through increased insulin release from the pancreatic beta cells, possibly by altering intracellular calcium.
Acknowledgements
The authors thank Al-khayat AH for identification of the plant material. INS-1E cells were kindly provided by Pierre Maechler, University of Genève.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alaadin AM Al-Khateeb EH, Jäger AK (2007): Antibacterial activity of the Iraqi Rheum ribes root. Pharm Biol 45: 688–690.
- Choi SZ, Lee SO, Jang KU, Chung SH, Park SH, Kang HC, Yang EY, Cho HJ, Lee KR (2005): Antidiabetic stilbene and anthraquinone derivatives from Rheum undulatum. Arch Pharm Res 28:1027–1030.
- Davis PH (1967): Flora of Turkey and East Aegean Islands. Cullen J 2: 268–269.
- Evans WC (2002): Trease and Evans Pharmacognosy, 15th edn. London, W.B. Saunders, pp. 229–231.
- Ernandez-Chacon RH, Alvarez de Toledo G (1995): Cytosolic calcium facilitates release of secretory products after exocytotic vesicle fusion. FEBS lett 263: 221–225.
- HMSO (1993): British Pharmacopoeia, Vol 1. London, pp. 577–578.
- Kaszkin-Bettag M, Richardson A, Rettenberger R, Heger PW (2008): Long-term toxicity studies in dogs support the safety of the special extract ERr 731 from the roots of Rheum rhaponticum. Food Chem Toxicol 46: 1608–1618.
- Li MC, Lei LS, Liang DS (1999): Effect of Ganoderma polysaccharide on intracellular free calcium in murine peritoneal macrophage. Chin Pharm J 34: 805–807.
- Li MC, Lei LS, Wang QB, Liang DS (2001): Effect of Ganoderma polysaccharide on intracellular free calcium and intracellular pH in murine T cells. Chin Pharmacol Bull 17: 167–170.
- Li JM, Che CT, Lau CB, Leung PS, Cheng CH (2007): Desoxyrhaponticin (3,5-dihydroxy-49-methoxystilbene 3-O-beta-d-glucoside) inhibits glucose uptake in the intestine and kidney: In vitro and in vivo studies. J Pharmacol Exp Ther 320: 38–46.
- Mericli AH, Tuzlaci E (1990): Constituents of Rheum ribes. Fitoterapia 61: 375.
- özbek H, Ceylan E, Kara M, özgökçe F, Koyuncu M (2004): Hypoglycemic effect of Rheum ribes roots in alloxan induced diabetic and normal mice. Scand J Lab Anim Sci 31: 113–115.
- Suresh Babu K, Tiwari AK, Srinivas PV, Ali AZ, China Raju B, Rao JM (2004): Yeast and mammalian β-glucosidase inhibitory constituents from Himalayan rhubarb Rheum emodi Wall. ex Meisson. Bioorg Med Chem Lett 14: 3841–3845.
- Tabata M, Sezik E, Honda G, Yesilada E, Fuki H, Goto K, Ikeshiro Y (1994): Traditional medicine in Turkey III. Folk medicine in East Anatolia, Van and Bitlis Provinces. Int J Pharmacog 32: 3–12.
- Tosun F, Akyuz Kizilay C (2003): Anthraquinones and flavonoids from Rheum ribes. J Fac Pharm Ankara 32: 31–32.