Abstract
Hydroalcoholic extract of Schima wallichii Choisy. (Ternstroemiaceae) bark (HESW) was investigated for its anti-inflammatory, antinociceptive, and antipyretic activities. The anti-inflammatory effects of the HESW were assayed by using carrageenan and dextran (acute model) induced paw edema and cotton pellet granuloma assay (chronic model) in experimental rats. Oral administration of HESW at the doses of 150 and 300 mg/kg caused dose-dependent inhibition of carrageenan and dextran induced inflammation. HESW at the doses of 150 and 300 mg/kg caused significant dose-dependent reduction of the granuloma tissue formation in experimental rats. The extract at the oral doses of 50 and 100 mg/kg body weight exhibited significant central and peripheral analgesic activity in acetic acid induced writhing test and hot-plate test respectively in experimental mice. Treatment with HESW at the oral doses of 150 and 300 mg/kg body weight significantly reduced the yeast-provoked elevated body temperature in experimental rats in a dose-dependent manner.
Introduction
Inflammatory diseases are a major cause of morbidity of the work force throughout the world. These have been called the “King of Human Miseries” (CitationChatterjee & Pal, 1984). Pain is an objectionable sensory and emotional incident associated with actual or potential tissue inflammation. Pyrexia or fever is caused as a secondary impact of inflammation (CitationKhan et al., 2007). Inflammation, pain, and fever are all associated with enhanced production of prostaglandins (CitationRang et al., 2003). Thus, most anti-inflammatory agents are expected to possess analgesic and antipyretic activity (CitationTripathi, 2001). Many commercially available products produce a dramatic symptomatic improvement in inflammatory conditions but cannot arrest the progress of the disease process and all of them share some of the common side effects namely hepatoxicity, nephrotoxicity, and gastrointestinal complications (CitationShah et al., 2006). Thus, there is an urgent need to develop a potential antipyretic pain killer with the least adverse effects (CitationSutradhar et al., 2006). In contrast, many traditional medicinal plants have been claimed to possess significant anti-inflammatory and analgesic activity without producing demonstrable adverse effects (CitationShah et al., 2006). The present study was undertaken to evaluate the anti-inflammatory, antipyretic, and analgesic activity of a traditional anti-inflammatory plant.
Schima wallichii Choisy. (Ternstroemiaceae) is a well known medicinal plant from the Sikkim Himalayan range. It is well known as “Chilauni” (Hindi), “Makrisal” (Bengali), “Alue-chilauni” (Nepali), “Sumbrang-kung” (Lepcha) in traditional medicine. The bark is used as antiseptic for cuts and wounds, anthelmintic, vermicide, mechanical irritant and to cure gonorrhea (CitationDewanjee et al., 2008). Though there is no scientific literature to support the anti-inflammatory and antinociceptive effect of Schima wallichii bark, the local people of East Sikkim use the bark for the treatment of fever and pain. The present paper attempted to explore scientifically the anti-inflammatory, antinociceptive, and antipyretic effects of hydroalcoholic extract of the bark of the said plant to substantiate the folklore claims.
Materials and methods
Plant material
The bark of Schima wallichii was collected from Majhitar, East Sikkim, India, in the month of April, 2006. The plant was authenticated by H.J. Chowdhury, Joint Director, Botanical Survey of India, Shibpur, Howrah, West Bengal. The voucher specimen (PPRL/DP/PT/JU/03/06) has been preserved in our laboratory for future reference.
Preparation of hydroalcoholic extract
The bark was dried under shade, pulverized into coarse powder, and extracted exhaustively by using 70% ethanol as a solvent in a Soxhlet extraction apparatus. The extract was evaporated under reduced pressure (40ºC, 0. 8 MPa) in a rotary vacuum evaporator (Buchi type, Mumbai, India) until all the solvent had been removed to give a semisolid extract (HESW) and finally lyophilized to ensure complete removal of solvent (yield 3% w/w).
Animals
Healthy adult Swiss albino mice (20-25 g) and Wister strain albino rats (140-160 g) of both sexes were used for the studies. Animals were procured from M.N. Ghosh Ltd., Kolkata, India. Animals were allowed to be acquainted for a period of 15 days in our laboratory environment prior to the experiment, housed in standard polypropylene cages, maintained under standard laboratory conditions (i.e. 12:12 h light and dark; at an ambient temperature of 25º ± 5ºC; 35-60% relative humidity); the animals were fed with standard diet (Hindustan Liver Ltd., Mumbai, India) and water ad libitum. The principles of laboratory animal care (CitationPHS, 1986) were followed and instructions given by our institutional animal ethical committee were followed throughout the experiment.
Anti-inflammatory activity in vivo models
Carrageenan and dextran induced paw edema in rats (acute models)
Pedal inflammation in rats was produced according to the method of CitationWinter and Porter (1957). Overnight fasted animals were divided into four groups of six animals each. Single subcutaneous injection of 0.1 mL of carrageenan (1% w/v) was given into the right paw of each rat under the subplantar aponeurosis (CitationGupta et al., 2003).
Group I received distilled water (2.0 mL/kg, orally) and served as inflamed control. Group II was treated with HESW (150 mg/kg, orally). Group III was treated HESW (300 mg/kg, orally). Group IV received standard drug indomethacin (10 mg/kg, orally).
The paw volume was measured by dipping the foot in the mercury bath of the plethysmograph up to the anatomical hairline on lateral malleolus and compared with control animals, which received only the vehicle. Measurement was done on h 0, 1, 2, 3, and 5 following carrageenan injection. The edema inhibitory activity was calculated according to the following formula. Percentage inhibition (%) = (Vc - Vt) / Vt × 100. Where Vc and Vt were mean edema volumes of control and treated groups, respectively.
In the dextran model, edema was induced by injection of dextran (0.05 mL, 1% w/v in saline) (CitationPerianayagam et al., 2006); indomethacin, 10 mg/kg (per oral (p.o.)), was used as a reference, the rest of the experiment is the same as described in the dextran model.
Cotton pellet granuloma in rats (sub-acute model)
This study was carried out by the cotton pellet implantation method in rats (CitationVetrichelvan & Jegadeesan, 2002). Under light ether anesthesia, sterile cotton pellets (Bengal Surgicals Limited, Kolkata) were implanted subcutaneously (10 mg) in the axilla and groin regions of the overnight fasted rats. Then experimental animals were divided into four groups of six animals each.
Group I served as control and received distilled water (2 mL/kg, orally) daily for 7 consecutive days. Group II was treated with HESW (150 mg/kg, orally) daily for 7 consecutive days. Group III was treated HESW (300 mg/kg, orally) daily for 7 consecutive days. Group IV received indomethacin (10 mg/kg, orally) daily for 7 consecutive days.
Then the animals were sacrificed on day 8, the cotton pellet removed, free from extraneous tissue and dried overnight at 60ºC and weighed. The percentage inhibition of dry weight of the granuloma were calculated and compared.
Antinociceptive activity
Acetic acid induced writhing test
The animals were divided into four groups of six mice each. Group I was treated with distilled water (2.0 mL/kg, orally) orally and served as normal control. Group II animals were treated with HESW (50 mg/kg, orally). Group III animals were treated with HESW (100 mg/kg, orally). Group IV animals were treated with standard drug paracetamol (50 mg/kg, orally).
One hour after extract and standard drug administration each animal was treated with a single intraperitoneal injection of 0.7% acetic acid (CitationAhmed et al., 2001). After the acetic acid injection, the number of abdominal constrictions (writhings) was counted for 10 consecutive minutes. The onset of writhing was also recorded.
Hot-plate test
The test was performed with the hot-plate maintained at a temperature of 50° ± 1°C (CitationWilson et al., 2003). The basal reaction time of all animals towards thermal heat was recorded. The animals which showed forepaw licking or jumping response within 6 seconds were selected for the study.
The animals were divided into four groups of six mice each. Group I was treated with distilled water (2 mL/kg, orally) and served as normal control. Group II animals were treated with HESW (50 mg/kg, orally). Group III animals were treated with HESW (100 mg/kg, orally). Group IV animals were treated with standard drug pentazocine (10 mg/kg, orally).
After the administration of test and standard compounds, the animals in all the four groups were individually exposed to the hot-plate maintained at 50° ± 1°C. The time taken in seconds for forepaw licking or jumping was taken as reaction time and recorded at 0, 30, 60, 90, 120 and 150 min. A cut-off period of 15 sec was observed to avoid damage to the paws.
Antipyretic activity
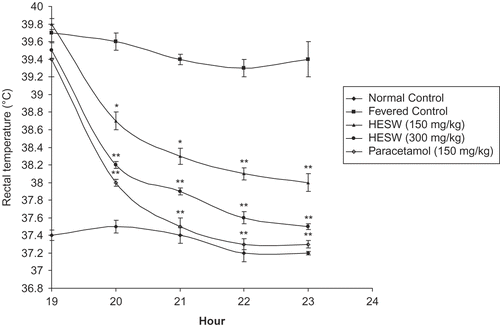
Fever was induced as per the method described by CitationSmith and Hambourger (1935). Initial rectal temperatures were recorded by insertion of a thermocouple to a depth of 2 cm into rectum. After measuring the basal rectal temperature, animals were given a subcutaneous injection of 10 mL/kg body weight of 15% w/v yeast suspended in 0.5% w/v methyl cellulose solution. Rats were then returned to their housing cages. After 19 h of yeast injection, the animals were divided into groups and were screened on the basis of elevated temperature and were treated as follows: Group I normal animals received distilled water (2 mL/kg, orally) orally and served as normal control. Group II fevered animals received distilled water orally and served as pyretic control. Group III fevered animals were treated with HESW (50 mg/kg, orally). Group IV fevered animals were treated with HESW (100 mg/kg, orally). Group V fevered animals were treated with standard drug paracetamol (150 mg/kg, orally).
The body temperature was measured at 19, 20, 21, 22, and 23 h (CitationDevi et al., 2003).
Statistical analysis
Data were statistically calculated by utilizing one way ANOVA and expressed as mean ± SEM followed by Dunnett’s t-test using computerized GraphPad InStat version 3.05, GraphPad Software, La Jolla, CA.
Results
The in vivo anti-inflammatory effect of the HESW was assayed by using carrageenan dextran-induced paw edema (acute model), and cotton pellet granuloma assay (sub-acute model). The administration of subplantar injection of carrageenan and dextran produced significant edema in the rat paws, reaching maximum at 4 h and 3 h, respectively. Oral administration of HESW caused dose-related inhibition of carrageenan and dextran induced inflammation. The extract at the doses of 150 and 300 mg/kg exhibited significant (p <0.05) anti-inflammatory activity with 15.28 and 16.58% inhibition respectively at 5 h after carrageenan administration (). The extract at the doses of 150 and 300 mg/kg also exhibited significant (p <0.01) anti-inflammatory activity at 5 h after dextran administration with maximum percentage inhibition of 12.89 and 13.95 respectively (). The HESW at the doses of 150 and 300 mg/kg exhibited 23.92% and 28.96% reduction (p <0.05) of the granuloma tissue formation, respectively, where as 37.48% inhibition (p <0.01) of granuloma was observed in standard drug indomethacin (10 mg/kg) treated group ().
Figure 1. Effect of hydroalcoholic extract of Schima wallichii bark on carrageenan induced paw edema in rats (n = 6). *p <0.05 compared with control group. **p <0.01 compared with control group.
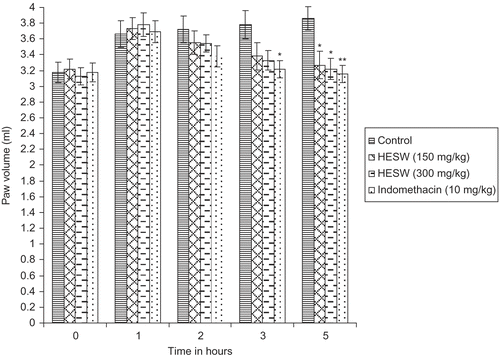
Figure 2. Effect of hydroalcoholic extract of Schima wallichii bark on dextran induced paw edema in rats (n = 6). *p <0.05 compared with control group. **p <0.01 compared with control group.
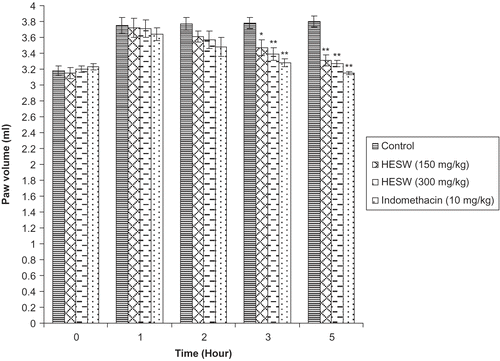
Table 1. Effect of hydroalcoholic extract of Schima wallichii bark on cotton pellet granuloma in rats.
HESW exhibited a significant decrease (p <0.01) of acetic acid induced writhing in mice by 30.82% and 50.65% at the doses of 50 and 100 mg/kg body weight, respectively. The effect was compared to that of standard drug, paracetamol (50 mg/kg) which showed inhibition of 64.76% (p <0.01) of writhing (). HESW also exhibited significant dose-dependent resistance (p <0.01) of force induced pain in 30 min onward, which persisted up to 120 min (). The extract exhibited maximum activity in 90 min. The experimental results are comparable with that of standard drug Pentazocine (10 mg/kg).
Table 2. Effect of hydroalcoholic extract of Schima wallichii bark on acetic acid induced writhing in mice.
Table 3. Evaluation of analgesic activity of hydroalcoholic extract of Schima wallichii bark by the hot-plate method.
Subcutaneous injection of yeast suspension significantly elevated (p <0.01) the rectal temperature substantially after 19 h of administration. Treatment with HESW significantly decreased the rectal temperature of the rats in a dose-dependent manner (). The antipyretic effect started as early as h 1 and the effect was maintained up to h 5, after drug administration. Experimental results were compared with standard antipyretic drug paracetamol (150 mg/kg).
Discussion and conclusions
In the present study anti-inflammatory activity of the HESW was evaluated with some acute and chronic in vivo models. The irritant effect of carrageenan is a result of activation of the kinin and complement cascades and consequently of the release of anti-inflammatory mediators such as vasoactive amines (histamine, 5-hydroxytryptamine and bradykinins) and eicosanoids (CitationLarsen & Henson, 1983; CitationVinegar et al., 1957). The release of these substances results in enhanced vascular permeability, thereby promoting accumulation of fluid in tissues that accounts for the edema (CitationWhite, 1999; CitationWilliams & Morley, 1973). Dextran induces paw edema mainly by releasing the histamine and 5-HT from the mast cells (CitationLo et al., 1982), Thus, carrageenan and dextran induced models are suitable test procedure to screen anti-inflammatory agents. In this study, HESW exhibited dose-dependent inhibitory effect in carrageenan and dextran induced paw edema and capable to attenuate the inflammation up to 5 h. The ability of the extract to reduce the edema volume suggests that the phytochemicals present in the extract may block or counteract the release of any of those mediators, alone or in combination. The cotton pellet induced granuloma test is reported to assess the ability of inflammatory effects of the proliferative phase of inflammation (CitationSelye, 1953). In the present study the HESW exhibited significant dose-dependent anti-inflammatory activity in the cotton pellet granuloma test, probably by inhibiting the increased number of fibroblasts, synthesis of collagen and mucopolysaccharides during granuloma tissue formation (CitationArrigoni-Martellie, 1977).
Acetic acid causes algesia by liberation of endogenous substances like PGE2 and PGF2α in the peritoneal fluid, which have been reported to be responsible for pain sensation (CitationTaesotikul et al., 2003). Thus, acetic acid is used widely to screen and study compounds for peripheral analgesic antinociceptive activity. The significant peripheral analgesic activity of HESW might be due to the effect of the extract on prostaglandin pathways. On the basis of the result of the acetic acid induced writhing test, it can be concluded that the extract might possess an analgesic activity and the mode of action might involve a peripheral mechanism. Hot-plate tests have been claimed as models for studying the central analgesic properties of a given substance (CitationZakaria et al., 2007). Hydroalcoholic extract significantly attenuates the thermally induced algesia by acting centrally. The HESW exhibited both central and peripheral antinociceptive activity and may be proven fruitful in future.
The present results show that the HESW possesses a significant antipyretic effect in yeast provoked elevation of body temperature in rats, and its effect is comparable to that of standard drug paracetamol. The antipyretic activity involved inhibition of the activity of prostaglandins synthesized within the central nervous system (CitationUzcátegui et al., 2004).
It is well known that the inflammatory responses are associated with augmented prostaglandin levels and result in pain, swelling and fever. In this experiment, HESW exhibited dose-dependent anti-inflammatory, analgesic, and antipyretic activities which may be associated with direct or indirect inhibition of prostaglandins synthesis.
Acknowledgement
The authors are grateful to Jadavpur University, India for providing facilities to conduct this experiment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmed M., Shikha HA, Sadhu SK, Rahman MT, Datta BK (2001); Analgesic, diuretic, and antiinflammatory principle from Scoparia dulcis. Pharmazie 56: 657–660.
- Arrigoni-Martellie E (1977): Inflammation and Antiinflammatory Spectrum Publication Inc., New York, pp. 119–120.
- Chatterjee GK, Pal SP (1984); Search for anti-inflammatory agents from Indian medicinal plants-A review. Indian Drugs 21: 413–419.
- Devi BP, Boominathan R, Mandal SC (2003); Anti-inflammatory, analgesic and antipyretic properties of Clitoria ternatea root. Phytother Res 14: 272–274.
- Dewanjee S, Maiti A, Majumdar R, Majumdar A, Mandal, SC (2008); Evaluation of antimicrobial activity of hydroalcoholic extract Schima wallichii bark. Pharmacologyonline 1: 523–528.
- Gupta M, Mazumdar UK, Sivakumar T, Amsi ML, Karki SS, Ambathkumar R, Manikandan L (2003); Evaluation of anti-inflammatory activity of chloroform extract of Bryonia laciniosa in experimental animal models. Biol Pharm Bull 26: 1342–1344.
- Khan A, Baki MA, Abdul M, Al-Bari A, Hasan S, Mosaddik MA, Rahman, MM, Haque ME (2007); Antipyretic activity of roots of Laportea crenulata Gaud in rabbit. Res J Med Sci 2(2): 58–61.
- Larsen GL, Henson PM (1983); Mediators of inflammation. Annu Rev Immunol 1: 335–359.
- Lo TN, Almeida AP, Beaven MA (1982); Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther 221: 261–267.
- Perianayagam JB, Sharma SK, Pillai KK (2006); Anti-inflammatory activity of Trichodesma indicum root extract in experimental animals. J Ethnopharmacol 104: 410–414.
- PHS (Public Health Service). (1986): Public Health Service Policy on Humane Care and Use of Laboratory Animals. Washington DC, U.S. Department of Health and Human Services, Office for Protection from Research Risks.
- Rang HP, Dale MM, Ritter JM, Moore PK (2003): Pharmacology, 5th edn. Churchill Livingstone, London, pp. 217–212.
- Selye H (1953); On the mechanism through which hydrocortocosterone affects the resistance of tissues to injury-An experimental study with granuloma pouch techniques. J Am Med Assoc 152: 1207–1213.
- Shah BN, Nayak BS, Seth AK, Jalalpure SS, Patel MA, Patel KN, Mishra AD (2006); Search for medicinal plants as a source of anti-inflammatory and anti-arthritic agents – A review. 2(6): 77–86.
- Smith PK, Hambourger WE (1935); The ratio of the toxicity of acetanilide to its antipyretic activity in rats. J Pharmacol Exp Ther 54: 346–351.
- Sutradhar RK, Rahman AM, Ahmad M, Bachar SC, Saha A, Guha SK (2006); Bioactive alkaloid from Sida cordifolia Linn. with analgesic and anti-inflammatory activities. Iranian J Pharmacol Ther 5(2): 1–10.
- Taesotikul T, Panthong A, Kanjanapothi D, Verpoorte R, Scheffer JJC (2003); Antiinflammatory, antipyretic and antinociceptive activities of Tabernaemontana pandacaqui Poir. J Ethnopharmacol 84: 31–35.
- Tripathi KD (2001): Essentials of Medical Pharmacology, 4th edn. Jaypee Brothers Medical Publishers, New Delhi, pp. 52–53.
- Uzcátegui B, ávila D, Suárez-Roca H, Quintero L, Ortega J, González B (2004); Anti-inflammatory, antinociceptive, and antipyretic effects of Lantana trifolia Linnaeus in experimental animals. Invest Clín 45: 317–322.
- Vetrichelvan T, Jegadeesan M (2002); Effect of alcoholic extract of Achyranthes bidentata Blume. on acute and sub acute inflammation Indian J Pharmacol 34: 115–118.
- Vinegar R, Truax JF, Selph JL, Jonhston PR, Venable AL, McKenzie RK (1957); Pathway to carrageenan induced inflammation in the hind limb of the rat. Fed. Proc 46: 118–126.
- White M (1999); Mediators of inflammation and inflammatory process. J Allergy Clin Immunol 103: 5378–5381.
- Williams TJ, Morley J (1973); Prostaglandins as potentiators of increased vascular permeability in inflammation. Nature 246: 215–217.
- Wilson SG, Bryant CD, Lariviere WR, Olsen MS, Giles BE, Chesler EJ Mogil JS (2003); The heritability of antinociception II: Pharmacogenetic mediation of three over-the-counter analgesics in mice. J Pharmacol Exp Ther 305: 755–764.
- Winter CA, Porter CC (1957); Effect of alteration in side chain upon anti-inflammatory and liver glycogen activity of hydrocortisone esters. J Am Pharm Assoc Sci 46: 515–519.
- Zakaria ZA, Kumar HG, Siti NH, Zaid M, Ghani MA, Hassan MH, Hazalin AMN, Khamis MM, Devi GR, Sulaiman MR (2007); Analgesic and antipyretic actions of Muntingia calabura leaves chloroform extract in animal models. Oriental Pharm Exp Med 7: 34–40.