Abstract
Adjuvant arthritis is one of the extensively used models of chronic inflammatory joint disorder such as rheumatoid arthritis. It is postulated that oxidative stress plays a pivotal role in rheumatoid arthritis. The current research was designed to examine the antioxidative effect of Gaultheria fragrantissima Wall. (Ericaceae) against complete Freund’s adjuvant induced arthritis. Arthritis was induced by single subcutaneous injection of complete Freund’s adjuvant (0.1 mL) into the plantar surface of the right hind paw of male Wistar rats. Gaultheria fragrantissima leaf extracts (100 and 200 mg kg−1day−1) and indomethacin (3 mg kg−1day−1) were administered orally for 14 days (from day 14 to day 28) after the adjuvant injection. The protective effect was evaluated by DPPH radical scavenging activity, alterations in paw volume, lipid peroxidation (measured in terms of MDA), antioxidant, enzymatic (SOD, CAT, GPx, GR and GST), nonenzymatic (GSH), and marker enzymes (AST, ALT, ALP and GGT) levels from liver and serum of adjuvant induced and treatment groups. These biochemical alterations were significantly (p < 0.05) ameliorated nearly to control values after administration of Gaultheria fragrantissima leaf extract to arthritic animals.
Introduction
Complete Freund’s adjuvant (CFA) is a formulation of heat-killed Mycobacterium tuberculosis that induces arthritis in Wistar rats which is a chronic model of inflammatory joint disorder characterized by analogous of pathobiochemical and pathophysiological changes as rheumatoid arthritis in human beings (CitationRes et al., 1988). Literature data have proved that rheumatoid arthritis is an autoimmune disease where reactive oxygen species (ROS) play a significant role in inflammation of joints (CitationBauerova & Bezek, 1999). In fact, ROS present a “paradox” (CitationDavies, 1995) in their biological functions: on one hand, control diseases through immune stimulation, cell signaling and, on the other, play a crucial role in apoptosis. ROS are generated through immunopotentiation where leucocytes such as macrophages and polymorphonuclear neutrophils play a fundamental role (CitationBogdan et al., 2000) and are responsible for differentiation and proliferation of T-cells (CitationDröge, 2002) which are liable for inflammation at synovium.
ROS such as superoxide radicals cause proliferation of fibroblasts, generate hydrogen peroxide (H2O2) and are involved in the activation of transcription factors such as NF kappa B (CitationDumont et al., 1999) that regulates the expression of cytokine genes including IL-2 and TNF-α resulting in inflammation in joints (CitationBaeuerle & Henkel, 1994). It has been reported that inflammation in synovial membrane is mediated by hydroxyl radical which lead to lipid peroxidation (LPO) in synovial membrane (CitationRowley et al., 1984) and several other tissues. ROS are effectively scavenged by cellular enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione S-transferase (GST) and non-enzymatic antioxidant glutathione (GSH) (özben, 1998). In the CFA induced oxidative stress model, either these antioxidant levels are decreased or generation of oxidants is increased (CitationHalliwell and Gutteridge, 1992).
In rheumatoid arthritis antioxidant systems are unable to control the generation of free radicals or oxidants, which is the root cause of oxidative stress observed in the present study. Though conventional treatment with steroids, NSAIDs and biological agents such as TNF-α, IL-1β antagonist can control the symptoms of acute arthritis, it is ineffective in chronic inflammation and prolonged use of those agents cause various side effects (CitationChandrasekaran et al., 2002). Research suggests that the use of complementary and alternative medicine, primarily herbal therapies, is a positive to treat arthritis (CitationCai et al., 2005). Therefore, the importance of antioxidants derived from plant origin has been increased greatly in recent years (CitationGülçin et al., 2004; CitationElmastaş et al., 2005). Hence, the study of antioxidant status during oxidative challenge can be used as a manifestation of defense against the development of degenerative inflammatory processes in tentative condition for therapeutic measures.
Gaultheria fragrantissima Wall. (GF) (Ericaceae) is an evergreen shrub, commonly known as Indian wintergreen, which is cultivated at high altitude. Traditionally, leaves of this plant are used in acute rheumatism, sciatic and intercostal neuralgia (CitationNadkarni, 1908). Pharmacological reports suggest that the plant possesses antidiabetic, anticancer, diuretic, hypothermic, analgesic, and antiepileptic activities (CitationBhakuni et al., 1971). Chemical investigation of leaves accounts the presence of beta-sitosterol, quercetin-3-galactoside and ursolic acid (CitationSamba Murthy & Rajendra Babu, 1970, Citation1972). Previous studies report that β-sitosterol has potential activity in rheumatoid arthritis (CitationMur et al., 2002) and it has been proved for its antioxidant and anti-inflammatory activity in carrageenan and cotton pellet induced edema in rats (CitationSingh, 1970). Ample data support the protective effect of the quercetin-3-galactoside (polyphenolic flavonoid), and ursolic acid (pentacyclic triterpenoid), against free radicals (CitationPereira et al., 2007; CitationJie, 1995). It has been well documented that ursolic acid has hepatoprotective (CitationSaraswat et al., 2000) and cardioprotective (CitationBalanehru & Nagarajan, 1992) activities and is also effective in rheumatoid arthritis (CitationIwu & Ohiri, 1980).
To the best of our knowledge, the effect of GF has never been reported in adjuvant induced arthritis. Therefore, by considering the phytochemical constituents, we evaluated the antioxidant potential of GF against CFA induced arthritic rats. In order to scrutinize the salubrious effect of GF, paw volume and some biochemical parameters including malondialdehyde (MDA) formation, liver marker enzymes, enzymatic (SOD, CAT, GPx, GR and GST) and non-enzymatic (GSH) antioxidant levels were determined.
Materials and methods
Chemicals
CFA and DPPH radical were obtained from Sigma Aldrich, St. Louis, MO, USA. Indomethacin from Cipla Ltd., Mumbai, India and all other chemicals were of analytical grade and highest purity.
Preparation of plant extract
G. fragrantissima Wall. leaf were procured from Survey of Medicinal Plants & Collection Unit, Ooty, Tamilnadu, India and authenticated (voucher numer of the specimen: PARC/2008/129) by Plant Anatomy Research Centre (PARC), Medicinal plant Research Unit, Chennai, India. Dried leaves were powdered and packed in Soxhlet apparatus and subjected to hot continuous percolation using ethanol (70% v/v) as solvent. The extract was concentrated under vacuum and dried in a vacuum desiccator (yield 9.3% w/w) and stored in an air-tight container for future use.
Animals
The study was performed on male albino rats of Wistar strain (average weight of 150-180 g), obtained from the Experimental Animal Care Centre, Vel’s College of Pharmacy, Chennai, India. The experimental protocol was approved by the Institutional Animal Ethical Committee (IAEC) of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, Ministry of Culture, Chennai. The animals were housed in standard laboratory conditions 12 ± 1 h day and night rhythm during the experimental period and they were given food and water supplied by Hindustan Lever Ltd., Mumbai, India under the trade name Gold Mohur rat feed and water ad libitum.
Experimental protocol
Animals were divided into five groups of six animals each group as follows:
Group I: Control rats received water and food.
Group II: Arthritic rats served as induced (arthritic syndrome was induced by single subcutaneous injection of 0.1 mL of CFA (10 mg of heat killed Mycobacterium tuberculosis mL−1 of paraffin oil) into the plantar surface of right hind paw, exactly on the first day of the experimental period).
Group III: Arthritic rats served as standard [aqueous suspension of indomethacin (3 mg kg−1day−1), orally for 14 days (from day 14 to day 28)] treated.
Group IV: Arthritic rats served as Test 1, treated with GF (100 mg kg−1day−1), orally for 14 days (from day 14 to day 28).
Group V: Arthritic rats served as Test 2, treated with GF (200 mg kg−1day−1) similar to Group IV.
Paw volumes of each group of animals were measured on days 15, 18, 21, 24, 27, and 28. The experimental period was terminated on day 29 and all the animals were sacrificed on the same day, by cervical decapitation; blood was collected without EDTA and centrifuged at 10,000 rpm for 10 min, the separated serum samples were used for marker enzymes estimation. The liver was immediately dissected out and the liver tissue homogenized in ice-cold 0.01 M Tris-HCL buffer, pH 7.4 to give a 10% homogenate was used for estimation of MDA and antioxidants.
Free radical scavenging activity
The free radical scavenging activity of GF extract was determined by the DPPH assay (CitationBlois, 1958). DPPH (2, 2-diphenyl-1-picryl hydrazyl) absorbs at 517 nm, but upon reaction with an antioxidant its absorption decreases while the percentage of inhibition increases. Briefly, 0.1 mM solution of DPPH in methanol was prepared, and 4 mL of this solution was added to 1 mL of sample solution in methanol at different concentrations. Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance (A) of the reaction mixture indicates higher free radical scavenging activity (% inhibition). The capability to scavenge the DPPH radical was calculated using the following equation.
where Acontrol = absorbance of DPPH solution without test sample and Asample = absorbance of DPPH solution with test sample.
Estimation of marker enzymes in serum
Aspartate transaminase (AST, E.C.2.6.1.1) and alanine transaminase (ALT, E.C.2.6.1.2) are estimated through measuring oxaloacetate and pyruvate produced, respectively, and measured at 564 nm, 37°C (CitationReitman & Frankel, 1957). Alkaline phosphatase (ALP, E.C.3.1.3.1) was assayed by using disodium phenyl phosphate as the substrate and Folin’s phenol reagent as blocking agent and the blue color intensity, measured at 640 nm (CitationKind & King, 1954). γ-Gluatamyl transferase (GGT, E.C.2.3.2.2) was estimated by using glutamyl-p-nitroanilide as substrate in ammediol-HCI buffer, pH 8.2. The increase in absorbance was measured at 25°C and 405 nm (CitationGabor, 1969).
Estimation of total protein
The total protein was estimated by the method of CitationLowry et al. (1951).
Estimation of lipid peroxidation in terms of MDA
The method described by CitationOhkawa et al. (1979) was used. The reaction mixture contained 0.1 mL of tissue homogenate, 0.2 mL of 8.1% sodium dodecyl sulphate (SDS), 1.5 mL of 20% acetic acid and 1.5 mL of 0.8% aqueous solution of thiobarbituric acid (TBA). The pH was adjusted with 1 N NaOH to 3.5. The mixture was finally made up to 4 mL with distilled water and heated at 95°C for 60 min in an oil bath. After cooling under tap water, 1 mL of distilled water and 5 mL of a mixture of n-butanol and pyridine (15:1) was added and the mixture was shaken vigorously on a vortex mixer. After centrifugation at 2200 rpm for 5 min the absorbance of the organic layer (upper layer) was measured immediately at 532 nm.
Estimation of GSH in liver
In the liver homogenate, reduced glutathione (GSH) activity was determined based on the reduction of Ellman’s reagent [5, 59-dithio-bis-(2-nitrobenzoic acid)]by SH groups to form 1 mole of 2-nitro-5-mercaptobenzoic acid per mole of SH (CitationEllman, 1959). The nitro-mercaptobenzoic acid has an intense yellow color and can be determined spectrophotmetrically. To 0.5 mL of 10% trichloroacetic acid, 6 mM disodium ethylene diaminetetraacetic acid, 0.5 mL of homogenate was added and shaken gently for 10-15 min. This was followed by centrifugation at 2,000 rpm for 5 min. The supernatant (0.2 mL) was mixed with 1.7 mL of 0.1 M potassium phosphate buffer (pH 8). At least a duplicate was made for each sample. 0.1 mL of Ellman’s reagent was added to each tube. After 5 min, the optical density was measured at 412 nm against a reagent blank.
Estimation of enzymatic antioxidants activity in liver
SOD activity in liver was assayed on the basis that enzyme inhibits the autooxidation of pyrogallol and the absorbance is measured spectrophotometrically at 470 nm (CitationMarklund & Marklund, 1997). CAT activity was measured by using hydrogen peroxide as substrate. The rate of decomposition of H2O2 was followed at 240 nm (CitationAebi, 1974). GPx was assayed by the method of CitationRotruck et al. (1973) using GSH as substrate and with H2O2 to assess the utilization of GSH by GPx. Final GSH content in reaction mixture was determined by Ellman’s reagent and absorbance was measured at 410-412 nm. GR activity was measured by the method of CitationSmith et al. (1988) by monitoring the rate of production of 5-thio-2-nitrobenzoic acid (TNB) from Ellman’s reagent at 412 nm, which is coupled with the GR reaction. GST activity was measured by the method of CitationHabig and Jakoby (1974). GST catalyzes the conjugation reaction with glutathione in the first step of mercapturic acid synthesis. The reaction mixture contained tissue homogenate, KH2PO4 buffer, EDTA, CDNB (1-chloro-2,4-dinitrobenzene) and GSH. The absorbance was measured at 340 nm.
Statistical analysis
The results were expressed as mean ± standard deviation (SD) for six animals in each group. Differences between groups were assessed by one-way analysis of variance (ANOVA) using the SPSS 13.0 software package for Windows. Post hoc testing was performed for inter-group comparisons using the least significance difference (LSD) test; the respective symbols in the tables have been considered statistically significant at p-values < 0.05.
Results
represents the adjuvant induced arthritis model. Group II developed periarticular erythema, edema which was reflected by increased (p < 0.05) paw volume in comparison to control (Group I) throughout the study period. Administration of GF, ethanolic leaf extract (100 mg kg−1, 200 mg kg−1) to test 1 (Group IV) and test 2 (Group V) respectively, reduced hind paw swelling significantly (p < 0.05) in comparison to Group II from day 15 to 28. Test 2 dose (200 mg kg−1) reduced paw edema comparatively more than that of test 1 dose (100 mg kg−1). The standard (Group III) drug, indomethacin (3 mg kg−1) led to a reduction in paw volume significantly (p < 0.05) when compared to Group II from day 15 to 28.
Table 1. Effect of CFA and GF on hind paw volume of rats.
shows that stable DPPH radical was effectively scavenged, and the values of DPPH scavenging effect (% inhibition) of different concentrations (50, 100, and 200 μg) of extract (GF) and α-tocopherol. As the values (% inhibition) are increased, the absorbance decreases with increased concentration of antioxidants. α-tocopherol was used as standard which has greater % inhibition value than GF extract.
Table 2. Effect of GF (extract) and α-tocopherol on DPPH radical.
As a consequence of arthritis induced by CFA, the levels of AST, ALT, ALP and GGT were significantly (p < 0.05) increased in Group II in comparison to Group I in serum (). After treatment with GF, these liver marker enzyme levels were reduced near to normal (p < 0.05) comparing with Group II. Similar results were found with Group III. The Group V showed better result than Group IV.
Table 3. Effect of CFA and GF on different liver marker enzymes in serum of rats.
shows the activity of antioxidant enzymes SOD, CAT, GPx, GR and GST levels are significantly (p < 0.05) decreased in Group II compared to Group I, while Group IV and V (test 1 and test 2, respectively) show, significant (p < 0.05) elevation of these enzymes levels which is in agreement with Group III compared to Group II.
Table 4. Effect of CFA and GF on different antioxidant enzymes in liver tissue of rats.
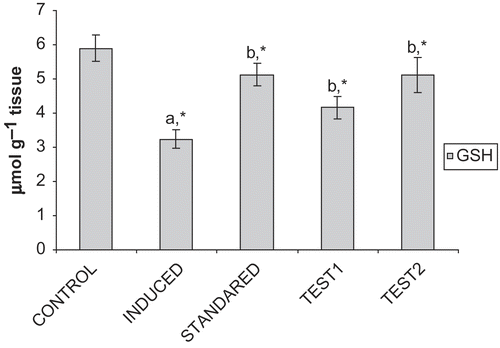
depicts that the level of GSH is reduced significantly (p < 0.05) in Group II, compared to Group I and after treatment with GF, the level is restored significantly (p < 0.05) near to normalcy both in Group IV and Group V. Elevation of GSH level is more in Group V than Group IV and in case of Group III the level is significantly (p < 0.05) increased compared to Group II.
Figure 1. Effect of CFA and GF on GSH levels in the liver of experimental animals. Values are expressed as mean ± SD for six animals. GSH levels are expressed as μmole g−1 tissue. Comparisons are made between: Group I (a) and Group II; Group II (b) and III, IV, and V. *Statistically significant (p < 0.05).
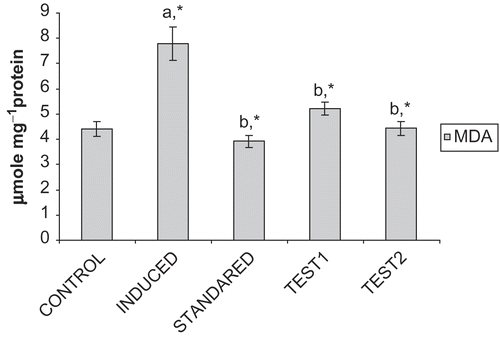
portrays that the levels of MDA, were reduced significantly (p < 0.05) in Group III, IV, V compared to Group II, which show significantly elevated levels, when compared with Group I.
Figure 2. Effect of CFA and GF on MDA levels in the liver of experimental animals. Values are expressed as mean ± SD for six animals. MDA levels are expressed as μmole 100mg−1protein. Comparisons are made between: Group I (a) and Group II; Group II (b) and III, IV, and V. *Statistically significant (p < 0.05).
The above results reveal that test 2 dose (Group V) significantly regulated the biochemical alterations compared to test 1 dose (Group IV) and hence it could be more effective in affording protection against the arthritic model.
Discussion
Rheumatoid arthritis is an autoimmune (CitationLevinson, 1994), chronic, relapsing, inflammatory disease characterized by swelling, pain, stiffness, infiltrating articular cartilage and proliferating synovial membrane. Animal model of rheumatoid arthritis is one of the best physiological conditions of oxidative stress which is relevant to the human disease (CitationBendele, 1999). Several researches reported that oxidative stress plays a predominant role in CFA induced rheumatoid arthritis which could be effectively managed by the phytochemical antioxidants (CitationVijayalakshmi et al., 1997; CitationGeetha et al., 1998). Hence, the present study was investigated on the antioxidant profile of Gaultheria fragrantissima in CFA induced arthritis in male Wistar rats. When CFA is injected in the right hind paw of rat, a non-infectious subacute polyarthritis, known as adjuvant arthritis, is induced (Geiler et al., 1974-Citation1975; CitationGlenn et al., 1977). As a result, periarticular tissue edema is formed which causes increased paw swelling in the arthritic animal with a change in ankle diameter (CitationMarylatha et al., 1998). In our study, the paw swelling was inhibited and paw volume was reduced after treatment with GF which may be due to its anti-inflammatory activity. The in vitro antioxidant activity of GF was appraised by stable DPPH radical in respect to α-tocopherol as standard. DPPH radical was efficiently scavenged by GF which proves its free radical scavenging potential.
ROS are generated when pathogens (bacteria) or immune complexes are ingested by activated phagocytic cells such as polymorphonuclear leucocytes or macrophages and form an oxidative burst that produce highly toxic ROS to kill those pathogens (CitationBabior, 2000). More oxygen is consumed during the formation of oxidative burst which is mediated by NADPH oxidases system resulting in the production of superoxide radical (CitationPithoncuri et al., 1998). Another strong oxidant, H2O2, is formed spontaneously from superoxide radical, and hydroxyl radical is formed by Fenton’s reaction between H2O2 and Fe+2 (ferrous ion) (CitationPillinger & Abramson, 1995). Apart from these ROS, several other oxidants such as nitric oxide radical, peroxynitrite radical are also similarly responsible for oxidative stress in adjuvant induced arthritis (CitationDel Carlo & Loeser, 2002; CitationJasin, 2005). ROS associated tissue damage can be measured by lipid peroxidation product. It has been well documented that ROS peroxidize the polyunsaturated fatty acid (PUFA) of cell membrane causing lipid peroxidation (CitationHeliovaara et al., 1994) of which MDA is the end product (CitationBernacka et al., 1992) and it plays an important physiological role in membrane destabilization (CitationMarnett, 1999). Literature data indicate that MDA is increased in CFA-induced oxidative stressed rats and it is due to lipid peroxidation of membrane (CitationRasoola & Varalakshmi, 2007) which is in line with our study. The treatment with GF decreased the MDA level and protected structural integrity of the cell membrane which might be due to its antiperoxidative effect.
Liver tissue damage was reviewed by measuring the levels of marker enzymes in the serum, since liver impairment is also a feature of adjuvant arthritis (CitationRainsford, 1982). The levels of AST, ALT, ALP, and GGT are also increased in the serum of CFA induced rats which may be due to increased lipid peroxidation, permeability of cell membrane or altered metabolism of these enzymes which is in agreement with the previous studies (CitationRajkapoor et al., 2007; CitationKatarína et al., 2006). The protective effect of GF may be due to the membrane stabilization action against the CFA induced oxidation. The levels of enzymatic antioxidants such as SOD and CAT were decreased in CFA induced rats (CitationMythilypriya et al., 2007) which may be due to their increased utilization in scavenging free radicals. SOD scavenges the superoxide radical and converts to H2O2, which is then converted to H2O by CAT (CitationMates, 2000) and, thereby, formation of hydroxyl radical is prevented. In our study, lipid peroxidation was inhibited, maybe due to increased levels of SOD and CAT after treatment with GF.
Other antioxidant enzymes such as GPx, GR, and GST also play a predominant role in scavenging free radicals. GPx is a selenoenzyme that catalyses the oxidation of GSH to glutathione disulfide (GSSG) and thereby scavenges H2O2 (CitationRister & Baehner, 1976) and, concomitantly, GR catalyses the reduction of GSSG to GSH (CitationBazzichi et al., 2002). GST is a detoxifying enzyme for endogeneous toxic products of LPO (CitationGupta et al., 1990). Previous reports suggest that GPX, GR and GST levels are decreased in CFA induced rats (CitationMythilypriya et al., 2007). The levels of this enzymatic antioxidant were found to be increased after treatment with GF, maybe due to its free radical scavenging activity. The level of non-enzymatic antioxidant GSH is also decreased in CFA induced rats (CitationFahim et al., 1995). The decreased activity of the GSH dependent antioxidant system might be due to accumulation of H2O2, exposure of high concentration of pathogens or immune complexes to T-lymphocyte which causes a significant rise in H2O2, decreases the intracellular GSH level (CitationLos et al., 1995). GF administration restored GSH level nearly to normal in CFA induced rats, perhaps due to increased levels of GPx and GR. Furthermore, severe GSH depletion is known to be associated with LPO (CitationKidd, 1997) and, in our study, LPO reduction paralleled GSH increment, after treatment with GF.
Several lines of studies indicated that phytosterols, flavonoids and pentacylic triterpenoids possess potent antioxidant and free radical scavenging potential. Previous reports suggested the presence of these constituents in GF and the constituents were structurally elucidated as β-sitosterol, quercetin-3-galactoside and ursolic acid (CitationSamba Murthy & Rajendra Babu, 1970, Citation1972). It has been well documented that β-sitosterol and quercetin-3-galactoside have antioxidant/anti-lipidperoxidative activities as they prevent the lipid peroxidation (CitationYokota et al., 2006; CitationBahorun & Trotin, 1994; CitationBahorun & Greiser, 1996) and also it has been reported that β-sitosterol refurbishes the ratio of glutathione/oxidized glutathione in RAW 264.7 macrophage cultures (CitationVivancos & Moreno, 2005) and decreases the inflammatory cytokines (CitationGupta et al., 1980). Several lines of evidence have shown that ursolic acid possesses antioxidant and anti-inflammatory properties (CitationWu et al., 1982; CitationKosuge et al., 1985). It has also been proved that ursolic acid has membrane stabilizing and radical scavenging activity (CitationHan et al., 1997; CitationBalanehru & Nagarajan, 1992). Recently, it has been reported that ursolic acid ameliorates adjuvant induced arthritis through distorted Th1/Th2 cytokine production (CitationAhmad et al., 2006) and also improves the GSH and CAT levels (Saravanan & Viswanathan, 2006; CitationKitani et al., 1999). Hence, it is suggested that presence of these phytoconstituents might be responsible for the bolstering of antioxidant defense system in CFA group, thereby precluding arthritis.
So, we can conclude that oral administration of Gaultheria fragrantissima could ameliorate the biochemical changes observed in CFA induced arthritic animals, presumably through the antioxidant, anti-radical and anti-peroxidative properties. However, the prospective studies to elucidate the exact mechanisms underlying the protective role of GF against CFA induced arthritis are highly warranted.
Acknowledgements
We are grateful to Dr. S. Rajan, Field Botanist, Survey of Medicinal Plants & Collection Unit, Ooty, Tamilnadu, India and Director, Professor P. Jayaraman, Plant Anatomy Research Centre, Chennai, Tamilnadu, India.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aebi H (1974): Catalase estimation. In: Berg Meyer HV, ed., Methods of Enzymatic Analysis. New York, Verlag Chemie, pp. 673–684.
- Ahmad SF, Khan B, Bani S, Suri KA, Satti NK, Qazi GN (2006): Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol Res 53: 233–240.
- Babior BM (2000): Phagocytes and oxidative stress. Am J Med 109: 33–44.
- Baeuerle PA, Henkel T (1994): Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179.
- Bahorun T, Trotin F (1994): Antioxidant activities of Crataegus monogyna extracts. Planta Med 60: 323–326.
- Bahorun T, Greiser B (1996): Oxygen species scavenging activity of phenolic extracts from Hawthorn fresh plant organs and pharmaceutical preparations. Arzneim Forsh/Drug Res 46: 1086–1089.
- Balanehru S, Nagarajan B (1992): Intervention of adriamycin induced free radical damage. Biochem Int 28: 735–744.
- Bauerova K, Bezek S (1999): Role of reactive oxygen and nitrogen species in etiopathogenesis of rheumatoid arthritis. Gen Physiol Biophys 18(Sp.Iss.): 15–20.
- Bazzichi L, Ciompi ML, Betti L, Rossi A, Melchiorre D, Fiorini M, Giannaccini G, Lucacchini A (2002): Impaired glutathione reductase activity and levels of collagenase and elastase in synovial fluid in rheumatoid arthritis. Clin Exp Rheumatol 20: 761–766.
- Bendele A (1999): Animal models of arthritis: Relevance to human disease. Toxicol Pathol 27: 134–142.
- Bernacka K, Sierakowsky S, Klimiuk PA, Chwiecko J (1992): Concentration of malondialdehyde in the serum of patients with rheumatoid arthritis. Folia Histochem Cytobiol 30: 203–204.
- Bhakuni DS, Dhar ML, Dhar MM, Dhawan BN, Gupta B, Srimal RC (1971): Screening of Indian plants for biological activity: Part III. Indian J Exp Biol 9: 91–102.
- Blois MS (1958): Antioxidant determinations by the use of a stable free radical. Nature ( London) 181: 1199–1200.
- Bogdan C Rollinghoff M, Diefenbach A (2000): Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 12: 64–76.
- Cai X, Zhou H, Wong YF, Xie Y, Liu ZQ, Jiang ZH, Bian ZX, Xu HXi, Liu L (2005): The comparative study of Sprague–Dawley and Lewis rats in adjuvant-induced arthritis. Biochem Biophys Res Commun 337: 586–594.
- Chandrasekaran S, Anilkumar S, Jamuna S (2002): Complementary and alternative drug therapy in arthritis. J Assoc Physicians India 50: 225–227.
- Davies KJA (1995): Oxidative stress: The paradox of aerobic life. Biochem Soc Symp 61: 1–31.
- Del Carlo JrM, Loeser RF (2002): Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum 46: 394–403.
- Dröge W (2002): Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95.
- Dumont A, Hehner SP, Hofmann TG, Ueffing M, Dröge W, Schmitz ML (1999): Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-κB. Oncogene 18: 747–757.
- Ellman GL (1959): Tissue sulfhydryl groups. Arch Biochem Biophys 17: 214–226.
- Elmastaş M, Gülçin İ, öztürk L, Gökçe İ (2005): Investigation of antioxidant properties of spearmint (Mentha spicata L.). Asian J Chem 17: 137–148.
- Fahim AT, Abd-el Fattah AA, Agha AM, Gad MZ (1995): Effect of pumpkin-seed oil on the level of free radical scavengers induced during adjuvant-arthritis in rats. Pharmacol Res 31: 73–79.
- Gabor S (1969): A kinetic photometric method for serum γ-glutamyl trnspeptidase. Clin Chem 15: 124–136.
- Geetha T, Varalakshmi P, Marylatha R (1998): Effect of triterpenes from Crateva nurvala stem bark on lipid peroxidation in adjuvant induced arthritis in rats. Pharmacol Res 37: 191–195.
- Geiler VG, Keitel W, Franke A (1974-1975): Vergleichende histologie der adjuvans-arthritis bei ratten, mausen und hamstern. Allerg Immunol 20-21: 251–252.
- Glenn EM, Bowman BJ, Rohloff NA, Seely RJ (1977): A major contributory cause of arthritis in adjuvant-inoculated rats: Granulocytes. Agents Actions 7: 265–282.
- Gülçin İ, şat İG, Beydemir ş, Küfrevioğlu Öİ (2004): Evaluation of the in vitro antioxidant properties of extracts of broccoli (Brassica oleracea L.). Ital J Food Sci 16: 17–30.
- Gupta S, Medh RD, Leal T, Awasthi YC (1990): Selective expression of the three classes of glutathione S-transferase isoenzymes in mouse tissues. Toxicol Appl Pharmacol 104: 533–542.
- Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP (1980): Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med 39: 157–163.
- Habig WH, Jakoby WB (1974): Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139.
- Halliwell B, Gutteridge JMC, Cross CE (1992): Free radicals, antioxidant and human disease: Where are we now? J Lab Clin Med 119: 598–620.
- Han SK, Ko YI, Park SJ, Jin IJ, Kim JM (1997): Oleanolic acid and ursolic acid stabilize liposomal membranes. Lipids 32: 769–773.
- Heliovaara M, Knekt P, Aho K, Aaran RK, Alfthan G, Aromaa A (1994): Serum antioxidants and risk of rheumatoid arthritis. Ann Rheum Dis 53: 51–53.
- Iwu MM, Ohiri FC (1980): Anti-arthritic triterpenoids of Lonchocarpus cyanescens Benth. Can J Pharm Sci 15: 39–42.
- Jasin HE (2005): Mechanisms of tissue damage in rheumatoid arthritis. In: Koopman WJ, Moreland LW, eds., Arthritis and Allied Conditions a Textbook of Rheumatology. Fifteenth edition. Philadelphia, Lippincot Williams and Wilkins, pp. 1141–1164.
- Jie Liu (1995): Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol 49: 57–68.
- Katarína B, Silvester P, Olľga O, Denisa K, Danica M (2006): Association between tissue gamma-glutamyltransferase and clinical markers of adjuvant arthritis in Lewis rats. Neuroendocrinol Lett 27(Suppl.2): 172–175.
- Kidd PM (1997): Glutathione: Systemic protectant against oxidative and free radical damage. Altern Med Rev 47: 359–370.
- Kind PR, King EJ (1954): Estimation of plasma phosphatase by determination of hydrolysed phenol with amino antipyrine. J Clin Pathol 7: 322–326.
- Kitani K, Kanai S, Ivy GO, Carrillo MC (1999): Pharmacological modifications of endogenous antioxidant enzymes with special reference to the effects of deprenyl: A possible antioxidant strategy. Mech Ageing Dev 111: 211–221.
- Kosuge T, Yokota M, Sugiyama K, Mure T, Yamazawa H, Yamamoto T (1985): Studies on bioactive substances in crude drugs used for arthritic diseases in traditional Chinese medicine. III. Isolation and identification of anti-inflammatory and analgesic principles from the whole herb of Pyrola rotundifolia L. Chem Pharm Bull 33: 5355–5357.
- Levinson SS (1994): Humoral mechanisms in autoimmune disease. J Clin Immunoassay 17: 72–84.
- Los M, Schenk H, Hexel K, Baeuerle PA, Dröge W, Schulzeosthoff K (1995): IL-2 gene expression and NF-κB activation through CD28 requires reactive oxygen production by 5-lipoxygenase. Embo J 14: 3731–3740.
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951): Protein measurement with Folin Wu reagent. J Biol Chem 193: 265–275.
- Marklund S, Marklund G (1997): Involvement of superoxide anion radical in the autooxidation of pyrogallol and a convenient assay of superoxide dismutase. Eur J Biochem 47: 469–474.
- Marnett LJ (1999): Lipid peroxidation-DNA damage malonaldialdehyde. Mutat Res 424: 83–95.
- Marylatha R, Geetha T, Varalakshmi P (1998): Effect of Vernonia cinerea Less flower extract in adjuvant-induced arthritis. Gen Pharmacol 31: 601–606.
- Mates M (2000): Effect of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153: 83–104.
- Mur E, Hartig F, Eibl G, Schirmer M (2002): Randomized double blind trial of an extract from the pentacyclic alkaloid-chemotype of uncaria tomentosa for the treatment of rheumatoid arthritis. J Rheumatol 29: 678–681.
- Mythilypriya R, Palanivelu S, Panchanatham S (2007): Restorative and synergistic efficacy of Kalpaamruthaa, a modified Siddha preparation, on an altered antioxidant status in adjuvant induced arthritic rat model. Chem Biol Interact 168: 193–202.
- Nadkarni KM (1908): Gaultheria fragrantissima Wall. The Indian Materia Medica. Bombay Popular Prakashan, Mumbai, India, pp. 570–571.
- Ohkawa H, Ohishi N, Nagi K (1979): Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L (2007): Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol 45: 2287–2295.
- Pillinger MH, Abramson SB (1995): The neutrophil in rheumatoid arthritis. Rheum Dis Clin North Am 21: 691–714.
- Pithoncuri TC, DeMelo MP, Palanch AC, Miyasaka CK, Curi R (1998): Percentage of phagocytosis, production of O2·–, H2O2 and NO and antioxidant enzyme activities of rat neutrophils in culture. Cell Biochem Funct 16: 43–49.
- Rainsford KD (1982): Adjuvant polyarthritis in rats: Is this a satisfactory model for screening arthritic drugs? Agents Actions 12: 452–458.
- Rajkapoor B, Ravichandran V, Anbu J (2007): Effect of Bauhinia variegate on complete Freund’s adjuvant induced arthritis in rats. J Pharmacol Toxicol 2: 465–472.
- Rasoola M, Varalakshmi P (2007): Protective effect of Withania somnifera root powder in relation to lipid peroxidation, antioxidant status, glycoproteins and bone collagen on adjuvant-induced arthritis in rats. Fund Clin Pharmacol 21: 157–164.
- Reitman S, Frankel S (1957): Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transminases. Am J Clin Pathol 28: 56–63.
- Res PC, Schaar CG, Breedveld FC, van Eden W, van Embden JD, Cohen IR (1988): Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet 2(8609): 478–480.
- Rister M, Baehner RL (1976): The alteration of superoxide dismutase, catalase, glutathione peroxidase, and NAD(P)H cytochrome c reductase in guinea pig polymorphonuclear leukocytes and alveolar macrophages during hyperoxia. J Clin Invest 58: 1174–1184.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973): Selenium: Biochemical role as a component of glutathione peroxidase. Science 179: 588–590.
- Rowley D, Gutteridge JM, Blake D, Farr M, Halliwell B (1984): Lipid peroxidation in rheumatoid arthritis: Thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci ( Lond) 66: 691–695.
- Samba Murthy K, Rajendra Babu M (1970): Chemical investigation of the leaves of Gaultheria fragrantissima Wall. (Ericaceae). Indian J Pharm 32: 176–178.
- Samba Murthy K, Rajendra Babu M (1972): Chemical investigation of the leaves of Gaultheria fragrantissima Wall. (Ericaceae). Indian J Pharm 34: 125–128.
- Saraswat B, Visen PKS, Agarwal DP (2000): Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother Res 14: 163–166.
- Saravanan R, Viswanathan P (2006): Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart. Pharmacol Reports 58: 41–47.
- Singh N (1970): A pharmacological study of Cyperus rotendus. Indian J Med Res 58: 103–109.
- Smith IK, Vierheller TL, Thorne CA (1988): Assay of glutathione reductase in crude tissue homogenates using 5, 59-dithiobis (2-nitrobenzoic acid). Anal Biochem 175: 408–413.
- Vijayalakshmi T, Muthulakshmi V, Sachdanandam P (1997): Salubrious effect of Semecarpus anacardium against lipid peroxidative changes in adjuvant arthritis in rats. Mol Cell Biochem 175: 65–69.
- Vivancos M, Moreno JJ (2005): beta-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radic Biol Med 39: 91–97.
- Wu JW, Lee MH, Ho CT, Chang SS (1982): Elucidation of the chemical structures of natural antioxidants isolated from rosemary. J Am Oil Chem Soc 59: 339–345.
- Yokota J, Takuma D, Hamada A, Onogawa M, Yoshioka S, Kusunose M, Miyamura M, Kyotani S, Nishioka Y (2006): Scavenging of reactive oxygen species by Eriobotrya japonica seed extract. Biol Pharm Bull 29: 467–471.
