Abstract
Shankhpushpi is a popular medicinal plant in the Ayurvedic system of medicine for treating mental disorders. Convulvulus pluricaulis Choisy. (Convulvulaceae) and Evolvulus alsinoides Linn. (Convulvulaceae) are used as Shankhpushpi by Ayurvedic practitioners. Ethanol extract of the aerial parts of both these drugs was evaluated for central nervous system (CNS) activity. The ethanol extract was fractionated into ethyl acetate and aqueous fractions and was tested in experimental models employing rats and mice. Elevated plus maze test, open field exploratory behavior and rotarod performance experiments were undertaken to observe influence on CNS. The extracts were also studied for their in vitro antioxidant potential to correlate their anxiolytic activity. In the elevated plus maze, ethyl acetate fractions of both the drugs at 100 mg/kg per oral showed an anxiolytic effect as evidenced by increase in the time spent in open arms and the number of open arm entries, compared to the control group. The open field exploratory behavior was also increased on administration of the ethyl acetate fractions (100 mg/kg p.o.) of both the drugs. The ethyl acetate fractions at doses of 200 mg/kg p.o. significantly reduced the neuromuscular coordination indicative of the muscle relaxant activity at a higher dose in both the drugs. The aqueous fractions of both the drugs were devoid of the above pharmacological actions at similar doses. Diazepam (1 mg/kg i.p.) was used as a standard in all the animal models studied. The present study provides scientific support for the anxiolytic and antioxidant activities of extracts of Evolvulus alsinoides and Convulvulus pluricaulis and substantiates the traditional claims for the usage of these drugs in stress-induced disorders.
Introduction
Modern lifestyles have resulted in stress-related disorders, and various approaches, for example, yoga, meditation and anti-stress drugs, are used to counteract aversive stress effects. Plant drugs have come to the rescue to mankind in many ailments and may offer satisfactory solutions to stress-induced perturbations. The importance of plants acting on the central nervous system (CNS) has been reviewed (CitationCarlini, 2003), emphasizing the role of adaptogens from plant origins. Rasayana is a clinical speciality of Ayurveda which prevents disease and counteracts the aging process by optimization of homeostasis and thereby rejuvenating the body (CitationAuddy et al., 2003). Medhya rasayana is a category of rasayana which rejuvenates, maintains, and potentiates intellect and memory.
Shankhpushpi is considered as “medhya rasayana” in Ayurvedic texts. Shankhpushpi of Ayurvedic Pharmacopoeia of India consists of the whole plant of Convulvulus pluricaulis Choisy (Convulvulaceae) (syn; Convulvulus microphyllus Sieb. ex Spreng) (MHFW, Citation2001a). Plants other than Convuluvulus pluricaulis are used as sources of drug in different parts of the country and Evolvulus alsinoides Linn. (EA) (Convulvulaceae) is also used as shankhpushpi by some practitioners. Other plants, e.g., Clitorea ternatea Linn. (Papilionaceae) and Canscora decussata Schult. (Gentianaceae) are also used as Shankhpushpi by some practitioners (CitationGupta et al., 2005). Whatever is the source, the drug finds use for its therapeutic effect on CNS disorders like insanity, epilepsy, nervous debility and memory enhancement (CitationChatterjee, 1990). Many formulations containing shankhpushpi as a single drug or in combination with other drugs are available in Indian markets and shankhpushpi is vigorously advertised for memory enhancement in print and electronic media in India.
To explore the scientific basis for the use of shankhpushpi, it was considered worthwhile to investigate various plants, which are used as shankhpushpi for their action on CNS. E. alsinoides is an important plant that has been well documented in Ayurveda for its therapeutic values. The plant is found throughout India, in the plains and to 6,000 ft in the Himalayas. Recent pharmacological studies on leaves and the whole plant of E. alsinoides have indicated anti-ulcer (CitationAsolkar et al., 1992), immunomodulatory (CitationLilly et al., 2003; CitationGanju et al., 2003), cytoprotective (CitationBhatnagar et al., 2000), adaptogenic and antiamnesic properties (CitationSiripurapu et al., 2005) and in vitro experiments (CitationAuddy et al., 2003) have revealed the antioxidant properties of E. alsinoides. Presence of water-soluble alkaloids betaine and evolvine has been reported. Evolvine (C12H17NO2) has been isolated and characterized as hydrochloride, oxalate, carbonate and mercury salt. A sterol and stearic, oleic and linolenic acids have also been isolated from the fatty residue. A good quantity of magnesium phosphate has been obtained from the aqueous extract (CitationVaradan et al., 1958). Some proteins, amino acids, and phenolic compounds are reported in E. alsinoides (CitationHanda & Kapoor, 1999).
Convulvulus pluricaulis is a prostrate, sub-erect, spreading, hairy, perennial herb with a woody rootstock, found throughout India on plains (CitationGupta et al., 2005). CitationUpadhyay (1986) studied the therapeutic role of Ayurvedic herbs in mental disorders and classified C. pluricaulis as a brain tonic. C. pluricaulis has been reported to exhibit antidiabetic (CitationAlam et al., 1990), anti-anxiety (CitationDandiya, 1990), tranquilizing (CitationHanda, 1994) and anti-ulcer properties (CitationSairam et al., 2001). C. pluricaulis is also reported to have hypolipidemic activities (CitationChaturvedi et al., 1995) and is useful in hyperthyroidism (CitationPanda & Kar, 2001). In phytochemical investigations on C. pluricaulis, an alkaloid (shankhpushpine), flavonoids, and inorganic salts (e.g. potassium chloride) have been reported. Two bases have been isolated. Base A (C5H11N02) depressed the blood pressure of an anesthetized dog, stimulated the isolated rat ileum and had temporary inhibitory action on pithed frog’s heart. Base B (C5H9NO2) was devoid of such pharmacological action (CitationRakhit & Basu, 1958).
The present study investigated the effects of E. alsinoides and C. pluricaulis, both of which are regarded as shankhpushpi acting on the central nervous system in rodents. Furthermore, the extracts were studied for their in vitro antioxidant potential to correlate their anxiolytic activity.
Materials and methods
Plant material
Aerial parts of E. alsinoides and C. pluricaulis were collected from Bhapel, a village in Sagar district, Madhya Pradesh, India, in the months of January to March 2006. The herbs were identified by Dr. Pradeep Tiwari, Department of Botany, Dr. Hari Singh Gour University, Sagar and preserved with voucher specimen numbers HBD/12030 and HBD/11015 for E. alsinoides and C. pluricaulis respectively in the herbarium of the institute.
Preparation of extracts
Aerial parts of E. alsinoides and C. pluricaulis were shade-dried at room temperature. The shade-dried plant material was coarsely powdered and subjected to extraction with petroleum ether in a Soxhlet apparatus. The defatted marc of both the drugs was subjected to ethanol (95%) extraction. The ethanol extract was suspended in distilled water and fractionated with ethyl acetate (Sigma Aldrich, St. Louis, MO, 98% pure) to get the ethyl acetate soluble fraction and the aqueous fraction. These fractions were utilized for the neuropharmacological investigation. Extraction was done as per the Ayurvedic Pharmacopoeia of India (CitationMHFW, 2001b). All chemicals used for the purpose were of analytical grade.
Chromatographic studies of extracts
Thin-layer chromatography
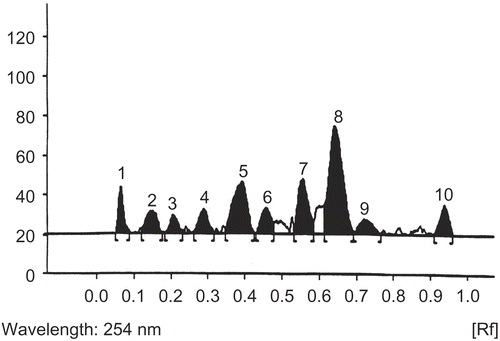
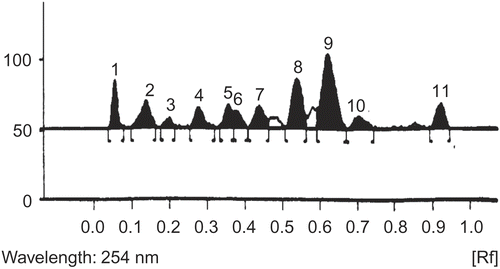
Out of the various solvent systems tried, chloroform: methanol: toluene (8:1:1) gave the best resolution (number of spots E. alsinoides = 14, C. pluricaulis = 11)
The detecting reagent was anisaldehyde in sulphuric acid followed by heating at 110°C for 5 min.
High Performance Thin Layer Chromatography (HPTLC) studies
Selection of HPTLC plates
Precoated and preactivated TLC plates (E. Merck No. 5548) of silica gel 60 F254 + 366, with the support of aluminum sheets having thickness of 0. 1 mm and size 20 × 20 cm, were cut smaller according to required dimensions (CitationAgrawal et al., 2004).
Sample preparation
Two grams of ethanol extract of EA and CP was weighed accurately and dissolved in 20 mL of ethanol. Solution was then refluxed for 30 min on a water bath at 60–70°C. The extract was cooled, filtered and finally volume made 20 mL with ethanol.
Application of sample
The extract samples were applied in the form of a band using CAMAG LINOMAT IV, an automatic sample application device, maintaining a band width 9 mm, space 9 mm, 15 sec/μL. The quantity of sample applied was 5–10 μL (CitationChopra et al., 2006).
HPTLC development
The following mobile phase was selected experimentally:
Chloroform: Glacial acetic acid: Methanol: Water (60:32:12:8).
The plates were developed by placing in a presaturated tank ( 12 cm height) with the mobile phase for 2 h. The plates were dried by evaporating the solvent either at room temperature or by spraying hot air by air dryer. The HPTLC densitograms are shown in and .
Animals
Sprague-Dawley rats (180-200 g) and Swiss albino mice (25-30 g) of either sex were used for the study. The animals were housed in groups of six in polypropylene cages, under standard laboratory conditions of temperature (25 ± 2°C), lighting (0800–2000 h) and relative humidity (50 ± 5%). The animals had free access to standard pellet chow (Brooke Bond-Lipton, India) and water. The animals were acclimatized for a minimum period of 7 days. Experiments were conducted between 0900 and 1400 h. The experimental protocol was approved by the Institutional Animals Ethics Committee (IAEC) and care of laboratory animals was taken as per CPCSEA guidelines (Reg. No. 379/01/ab/CPCSEA).
Drugs and chemicals
Diazepam (Calmpose, 10 mg/2 mL injection, Ranbaxy Laboratories, India) was used as the reference drug in all the animal models studied. It was dissolved in normal saline for i.p. injection. For in vitro antioxidant activity riboflavin, nitro blue tetrazolium (NBT) and l-ascorbic acid were purchased from Sigma Aldrich, St. Louis, MO. EDTA was obtained from Himedia.
Administration of the extracts
Suspensions of the ethyl acetate and aqueous fractions were prepared in distilled water using Tween 80 (0.2% v/v) as the suspending agent. The extracts were administered in a dose of 100 and 200 mg/kg to rats by oral route, 45 min before the test procedures. Control groups were given only the vehicle (0.2% v/v Tween 80 solution) in volume equivalent to that of the plant extracts.
Assessment of anxiolytic activity
Elevated plus maze test
Anxiolytic activity was evaluated using the elevated plus maze (CitationPellow et al., 1985). The elevated plus maze (EPM) consisted of two open arms (50 × 10 cm) crossed with two closed arms (50 × 10 × 40 cm). The arms were connected together with a central square (10 × 10 cm). The apparatus was elevated to a height of 70 cm in a dimly illuminated room. The animals were divided into six groups containing six animals each. The ethyl acetate and aqueous fractions (100 mg/kg p.o.) of E. alsinoides and C. pluricaulis were administered 45 min before trial on the EPM in separate groups of animals. Control groups were given only the vehicle (0.2% v/v Tween 80 solution) in volume equivalent to that of the plant extracts. Diazepam (1 mg/kg i.p.) was used as the reference drug for comparison. One hour post-administration, each rat was placed individually at the center of the elevated maze. The number of entries in the open and closed arm of the elevated maze during a period of 5 min and the duration of stay in the open and the closed arm were noted (CitationSoman et al., 2004).
Open field test
This test was performed following the method described by CitationBronstein (1972). The open field apparatus was made of plywood and consisted of squares (61 × 61 cm). The entire apparatus was painted black except for 6 mm thick white lines, which divided the floor into 16 squares. The open field was lit by a 40 W bulb focusing on to the field from a height of about 100 cm. The entire room, except the open field was kept dark during the experiment. Each animal was centrally placed in the test apparatus for 5 min and the following behavioral aspects were noted:
Ambulation: this was measured in terms of the number of squares crossed by the animal;
Rearings: number of times the animal stood on its hind limbs;
Self-grooming: number of times the animal groomed facial region, and licked/washed/scratched various parts of its body;
Activity in center: number of central squares crossed by the animal; and,
Fecal droppings: number of fecal droppings excreted during the period.
The rats were divided into 6 groups containing six animals each. The ethyl acetate and aqueous fractions (100 mg/kg p.o.) of E. alsinoides and C. pluricaulis were administered 45 min before trial on the open field apparatus in individual groups of animals. Control groups were given only the vehicle (0.2% v/v Tween 80 solution) in volume equivalent to that of the plant extracts. Diazepam (1 mg/kg i.p.) was used as the standard drug for comparison.
Neuromuscular coordination–rotarod
The effect on motor coordination was assessed using a rotarod apparatus (CitationDunham & Miya, 1957). A day before the test, mice were trained to remain on the rotating rod ( 3 cm in diameter, 40 rpm). On the test day, mice were tested on the rotarod before and 45 min after the administration of vehicle, diazepam (1 mg/kg i.p.) or the ethyl acetate and aqueous fractions of E. alsinoides and C. pluricaulis (100 and 200 mg/kg p.o.). The number of seconds each mouse remained on the rotating wheel was recorded before and after the administration. The percentage reduction in the motor coordination was calculated from the readings obtained.
In vitro antioxidant activity – assay for superoxide radical scavenging activity
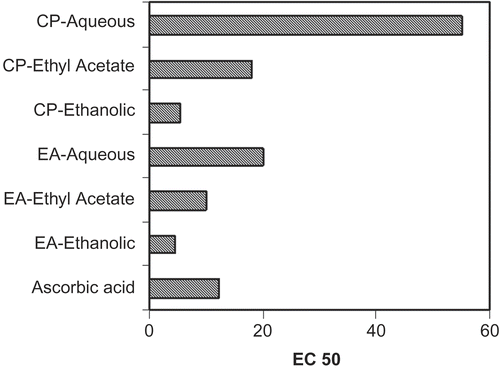
The assay was based on the capacity of the samples to inhibit blue formazan formation by scavenging the superoxide radicals generated in riboflavin-light-NBT system (CitationBeauchamp & Friedovich, 1971). The reaction mixture contained 50 mM phosphate buffer (pH 7.6), 20 μg riboflavin, 12 mM EDTA, 0.1 mg/3mL NBT, added in that sequence. The reaction was started by illuminating the reaction mixture with different concentration of samples. Immediately after illumination, the absorbance was measured at 590 nm and EC50 (effective concentration, required to inhibit NBT reduction by 50%) values were calculated from the dose-inhibition curves by graphical method (CitationSokmen et al., 2005). Ascorbic acid was used as positive control (CitationBagul et al., 2005). The ethanol extracts of the drugs as well as their ethyl acetate and aqueous fractions were evaluated for their free radical scavenging activity by this method ().
Statistical analysis
The data were expressed as mean ± SEM and statistically analyzed using one-way ANOVA followed by Dunnet’s test. P < 0.05 was considered to be statistically significant. F and P values and degrees of freedom were calculated for the readings obtained.
Results
Elevated plus maze test
The oral administration of the ethyl acetate fractions of E. alsinoides and C. pluricaulis (100 mg/kg p.o.) produced a significant increase (P < 0.5) in the time spent in the open arms as well as the number of entries in the open arm of the elevated plus maze indicating the anxiolytic activity of the drugs. Vehicle treated rats spent 27.33 ± 18. 39 s in the open arm. Rats treated with ethyl acetate fractions of E. alsinoides and C. pluricaulis spent 97.33 ± 3. 51 s and 95.66 ± 9. 95 s in the open arm respectively. The number of open arm entries also increased to 8.16 ± 1.57 and 8.50 ± 2.07 for E. alsinoides and C. pluricaulis, respectively, as compared to the vehicle (1.00 ± 0.44). The aqueous fractions (100 mg/kg p.o.) did not cause any significant change in the above parameters. The administration of diazepam (1.0 mg/kg i.p.) significantly increased (P < 0.01) the number of entries as well as the duration of stay in the open arms, as compared to the extract treated groups. F values were also calculated and found to be extremely significant. The results are given in .
Table 1. Effect of test fractions on exploratory activity of the rats in elevated plus maze apparatus.
Open field test
The oral administration of ethyl acetate fractions significantly increased the ambulatory activity (P < 0.5 for E. alsinoides and P < 0.01 for C. pluricaulis), rearings (P < 0.01 for E. alsinoides and P < 0.001 for C. pluricaulis), self-groomings (P < 0.5 for both the drugs) and activity in center (P < 0.01 for E. alsinoides and P < 0.001 for C. pluricaulis). The open field fecal droppings were significant in the case of E. alsinoides and comparable to vehicle in the case of C. pluricaulis. Diazepam also induced significant anxiolytic activity and the effects were found to be highly significant (P < 0.001) and more than that of the extracts. The aqueous fractions did not produce significant changes, indicating a lack of anxiolytic activity. F values were calculated and found to be extremely significant. The results are shown in .
Table 2. Effect of test fractions on open field exploratory behavior in rats.
Neuromuscular coordination–rotarod
Diazepam (1 mg/kg i.p.) and the ethyl acetate fractions (200 mg/kg p.o.) of E. alsinoides and C. pluricaulis caused significant reduction in the time spent on the rotarod, compared to control group (P < 0.001). The reduction in the performance on the rotarod before and after the experiment was 53.56 ± 6.76% and 69.56 ± 4.23% for groups treated with E. alsinoides and C. pluricaulis ethyl acetate fractions (200 mg/kg, p.o.) and 78.65 ± 3.79% for the diazepam-treated group as compared to the vehicle-treated control group (4.04 ± 1.33%). The ethyl acetate fractions of E. alsinoides and C. pluricaulis were devoid of significant effect on motor coordination at 100 mg/kg oral dose. The same was the case with the aqueous fractions (100 and 200 mg/kg, p.o.) of E. alsinoides and C. pluricaulis. These results indicate the muscle relaxant activity of the ethyl acetate fractions of E. alsinoides and C. pluricaulis at the higher dose of 200 mg/kg. Surprisingly, this is the first report about the muscle relaxant activity of both drugs. F and P values came out to be highly significant indicating the accuracy of the results. The results are shown in .
Table 3. Effect of test fractions on rotarod performance.
Antioxidant activity–superoxide radical scavenging activity
The assay was based on the capacity of the samples to inhibit blue formazan formation by scavenging the superoxide radicals generated in riboflavin-light-NBT system. The total ethanol extract and its ethyl acetate and aqueous fractions exhibited significant antioxidant activity, comparable to that of ascorbic acid, used as a positive control. The best superoxide radical scavenging activity was shown by the total extract followed by the ethyl acetate and aqueous fractions in both E. alsinoides and C. pluricaulis (). These preparations inhibited the development of color, produced during the reaction of superoxide with NBT by 79.97%, 74.82%, and 68.45% (E. alsinoides ethanol, ethyl acetate and aqueous fractions, respectively) and 88.77%, 77.95%, and 54.39% (C. pluricaulis ethanol, ethyl acetate, and aqueous fractions respectively). Moreover, the samples of total extract and its ethyl acetate and aqueous fractions suppressed superoxide radical release in a dose-dependent manner. Inhibition of NBT-reduction by superoxide in the presence of tested preparations increased with the increase of their concentrations.
Discussion
The question of reliability and validity is of prime importance in establishing experimental paradigms of practical predictable value. These factors assume further importance when animal models of human behavior and its perturbations are being used. The paradigms used in the present study have been subjected to thorough critical appraisal and validated as animal models of anxiety (CitationTreit, 1971; CitationFile, 1985, Citation1988; CitationLister, 1990; CitationKumar et al., 2000). Thus, in the open field and similar tests, when the animals are taken from their home cage and placed in a novel environment, they express their anxiety and fear by a decrease in ambulation, rearings, and other exploratory behaviors. Likewise, the elevated plus maze test is based on the principle that exposure to the maze leads to an approach conflict which is considerably stronger than that evoked by exposure to the enclosed part of the maze (CitationPellow et al., 1985). All these behaviors are increased by anxiogenic agents and attenuated by anxiolytics under identical experimental conditions (CitationKumar et al., 2000).
The findings of the present study indicate that ethyl acetate fractions of E. alsinoides and C. pluricaulis caused significant anxiolysis in the elevated plus maze test. Diazepam (1.0 mg/kg i.p.) significantly increased (P < 0.01) the number of entries as well as the duration of stay in the open arms, indicating anxiolytic activity. The ethyl acetate fraction of both the drugs was found to be effective. The aqueous fraction was devoid of such response in both the cases. Thus both the drugs came out to be good anxiolytics using EPM.
Anxiolytics have been shown to increase the open field ambulation, rearings, self-groomings and activity in the center, and a decrease in the number of fecal droppings is also apparent as a result of their administration. The anxiety induced by the open field conditions is attenuated by anxiolytic drugs (CitationKumar et al., 2000). The ethyl acetate fractions of both E. alsinoides and C. pluricaulis have shown sufficient anxiolytic activity. The aqueous fractions did not produce significant changes. C. pluricaulis proved to be a better anxiolytic than E. alsinoides in this model.
CitationVale et al. (1999) affirmed that the absence of interference with the motor coordination in the rotative bar discards the possibility of a muscular relaxing effect. In the present work, the ethyl acetate fractions of E. alsinoides and C. pluricaulis were devoid of significant effect on motor coordination at 100 mg/kg oral dose. The same was the case with the aqueous fractions (100 and 200 mg/kg p.o.) of E. alsinoides and C. pluricaulis. The ethyl acetate fractions of E. alsinoides and C. pluricaulis at the higher dose of 200 mg/kg were found to produce a significant reduction in the rotarod performance. Therefore, the observed reduction of the motor activity may be related to the muscle relaxant effect of the extracts.
Further, in the present study, the extracts were studied for their in vitro antioxidant potential to correlate their anxiolytic activity. This investigation of the total ethanol extract and its fractions was performed in a non-enzymatic system–NBT, riboflavin, and light. The assay was based on the capacity of the samples to inhibit blue formazan formation by scavenging the superoxide radicals generated in the riboflavin-light-NBT system. Superoxide radical is known to be very harmful to cellular components as a precursor of more reactive oxygen species (CitationHalliwell & Gutteridge, 1985). Free radicals and other reactive oxygen species are considered to be important causative factors in the development of diseases of aging such as neurodegenerative diseases, cancer and cardiovascular diseases (CitationAuddy et al., 2003). The total ethanol extract and its ethyl acetate and aqueous fractions exhibited significant antioxidant activity, comparable to that of ascorbic acid, used as a positive control. Preliminary phytochemical studies on the drugs showed the presence of flavonoids in the ethanol extract. This is in accordance with the findings that the antioxidant properties of flavonoids were affected mainly via scavenging of superoxide anions (CitationHaslem, 1966; CitationRice Evans et al., 1977). Thus the antioxidant potential of the extracts throws light on the cytoprotective effects of the extracts and usage in stress-induced disorders.
Thus, the present investigations on E. alsinoides and C. pluricaulis have led us to conclude that both the drugs possess significant anxiolytic effects, have effects on the neuromuscular coordination, and possess significant antioxidant potential.
Acknowledgements
The authors are grateful to the Director, B.R. Nahata Smriti Sansthan – Contract Research Centre, Mandsaur, Madhya Pradesh India for granting permission to carry out the in vivo studies. Alok Nahata is thankful to the University Grants Commission, New Delhi for providing a junior research fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agrawal H, Kaul N, Paradkar AR, Mahadik KR (2004): HPTLC method for guggulsterone II. Stress degradation studies on guggulsterone. J Pharm Biomed Ana 36: 23–31.
- Alam MM, Siddiqui MB, Hussain W (1990): Treatment of diabetes through herbal drugs in rural India. Fitoterpia 61: 240–242.
- Asolkar LV, Kakkar KK, Chakre OJ (1992): Second Supplement to Glossary of Indian Medicinal Plant with Active Principles. New Delhi, India, NISCAIR, pp. 1965–1985.
- Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B (2003): Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J Ethnopharmacol 84: 131–138.
- Bagul MS, Kanaki NS, Rajani M (2005): Evaluation of the free radical scavenging properties of two classical herbal formulations. Indian J Exp Biol 43: 732–736.
- Beauchamp C, Friedovich I (1971): Superoxide dismutase: improved assay and an assay applicable to polyacrylamide gels. Anal Biochem 44: 276–287.
- Bhatnagar M, Shukla SD, Jain S, Mundra A (2000): Cytoprotective effects of Shankhpushpi - an E. alsinoides preparation on Hippocampal cells in mice. Indian Drugs 37: 280–285.
- Bronstein PM (1972): Open field behaviour of the rat as a function of age: cross sectional and longitudinal investigations. J Comp Physiol Psychol 80: 335–341.
- Carlini EA (2003): Plants and the central nervous system. Pharmacol Biochem Behav 75: 501–512.
- Chatterjee A (1990): Treatise of Indian Medicinal Plants. New Delhi, India, Council for Scientific and Industrial Research, p. 327.
- Chaturvedi M, Mali PC, Dixit VP (1995): Hypolipidaemic effect of Convulvulus microphyllus in cholesterol fed gerbils. J Phytological Res 8: 153–155.
- Chopra S, Farhan JA, Khar RK, Motwani SK, Mahdi S, Iqbal Z, Talegaonkar S (2006): Validated high-performance thin-layer chromatography method for determination of trigonelline in herbal extract and pharmaceutical dosage form. Analytica Chimica Acta 577: 46–51.
- Dandiya PC (1990): The pharmacological basis of herbal drugs acting on the central nervous system. The Eastern Pharmacist 12: 39–47.
- Dunham NM, Miya TS (1957): A note on a simple apparatus for detecting neurologoical deficit in rats and mice. J Amer Pharm Assoc 46: 208–209.
- File SE (1985): Animal models for predicting clinical efficacy of anxiolytic drugs. Neuropsychobiology 13: 55–62.
- File SE (1988): How good is social interaction as a test of anxiety? In: Simon P, Soubrie P & Wildlocher D, eds., Selected Models of Anxiety, Depression and Psychosis. Basel, Karger, p. 151.
- Ganju L, Karan D, Chanda S, Shrivastava KK, Sawhney RC, Selvamurthy W (2003): Immunomodulatory effects of agents of plant origin. Biomed Pharmacother 57: 296–300.
- Gupta AK, Tandon N, Sharma M (2005): Quality Standards of Indian Medicinal Plants. New Delhi, Indian Council of Medical Research, p. 70.
- Halliwell B, Gutteridge JMC (1985): Free Radicals in Biology and Medicine, 2nd edn. Clarendon Press, Oxford, pp. 107–170, 627–630.
- Handa SS, Kapoor VK (1999): Pharmacognosy, 2nd edn. New Delhi, Vallabh Prakashan, pp. 237–239.
- Handa SS (1994): Rasayana Drugs. Pharma Times 26: 17–25.
- Haslem E (1966): Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J Nat Pro 59: 205–215.
- Kumar V, Singh RK, Jaiswal AK, Bhattacharya SK, Acharya SB (2000): Anxiolytic activity of Indian Abies pindrow Royle leaves in rodents: an experimental study. Indian J Exp Biol 38: 343–346.
- Lilly G, Dev K, Sudipta C, Srivastava KK, Sawhney RC, Selvamurthy W (2003): Immunomodulatory effects of agents of plant origin. Biomed Pharmacother 57: 296–300.
- Lister RG (1990): Ethologically based animal models of anxiety disorders. Pharmacol Ther 46: 321–340.
- MHFW (Ministry of Health, and Family Welfare) (2001a): The Ayurvedic Pharmacopoeia of India, Vol. 2. Delhi, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homeopathy, Controller of Publications, pp. 147–149.
- MHFW (2001b): The Ayurvedic Pharmacopoeia of India, Vol. 1. Delhi, Ministry of Health and Family Welfare, Department of Indian Systems of Medicine and Homeopathy, Controller of Publications, p. 94.
- Panda S, Kar A (2001): Inhibition of T3 production in levothyroxine-treated female mice by the root extract of Convulvulus pluricaulis. Horm Metab Res 33: 16–18.
- Pellow S, Chopin P, File SE, Briley M (1985): Validation of open/closed arms entries in an elevated plus maze as a measure of anxiety in rat. J Neurosci Meth 14: 149–167.
- Rakhit S, Basu NK (1958): Investigations on Convulvulus pluricaulis Chois. Part II. Ind J Pharm 20: 357–360.
- Rice Evans CA, Miller NJ, Paganga J (1977): Antioxidant properties of phenolic compounds. Trends in Plant Sci 2: 152–159.
- Sairam K, Rao CV, Goel RK (2001): Effect of Convulvulus pluricaulis Chois. on gastric ulceration and secretion in rats. Indian J Exp Biol 39: 350–354.
- Siripurapu KB, Gupta P, Bhatia G, Maurya R, Nath C, Patil G (2005): Adaptogenic and anti-amnesic properties of Evolvulus alsinoides in rodents. Pharmacol Biochem Behav 81: 424–432.
- Sokmen M, Angelova M, Krumova E, Pashova S, Ivancheva S, Sokmen A, Serkedjeva J (2005): In vitro antioxidant activity of polyphenol extracts with antiviral properties from Geranium sanguineum L. Life Sci 76: 2981–2993.
- Soman I, Mengi SA, Kasture SB (2004): Effect of leaves of Butea frondosa on stress, anxiety, and cognition in rats. Pharmacol Biochem Behav 79: 11–16.
- Treit D (1971): Animal models for the study of anti-anxiety agents: A review. Neurosci Behav Rev 9: 203–222.
- Upadhyay VP (1986): Therapeutic role of Ayurvedic herbs in mental disorders. Vedic Path 69: 24–29.
- Vale TG, Matos FJA, DeLima TCM, Viana GSB (1999): Behavioral effects of essential oils from Lippia alba (Mill). NE Brow chemotypes. J Ethnopharmacol 167: 127–133.
- Varadan KSS, Vaidyanathan TS, RamaRao MV (1958): Phytochemistry of Evolvulus alsinoides Linn. Ind J Pharm 20: 100–104.