Abstract
Seed extracts of three plant species that grow wild in the arid regions of Tunisia, Juniperus phoenicea L. (Cupressaceae), Pistacia atlantica Desf. (Anacardiaceae), and Oudneya africana R. Br. (Brassicaceae), were examined for antimicrobial activity against bacterial food pathogens. Aqueous extracts were prepared and then precipitated with methanol or acetone. Extracted acetone fractions (pH 7.2) showed powerful antimicrobial activity, especially against Listeria monocytogenes, Listeria innocua, and Listeria ivanovii (Gram-positive) and were also active against Gram-negative strains Escherichia coli and Pseudomonas aeruginosa. Extracts selected for high antimicrobial activity were stable in the presence of organic solvents (chloroform, hexane, acetonitrile, methanol, and acetone), and withstand thermal treatments up to 100°C for 30 min. L. monocytogenes LSD530 and E. coli ATCC 25922 appeared to be inhibited by Juniperus and Pistacia extracts with a minimum concentration of 1.56 and 3. 12 mg/mL, respectively. This study established the potential of medicinal plants growing wild in arid regions of Tunisia as a source of antimicrobial agents.
Introduction
The incidence of bacterial infections in humans is becoming a major concern in both the food and medical sectors worldwide. Several factors may explain this development. Over-use of antibiotics in agriculture and medicine has led to the emergence of highly resistant pathogenic microorganisms, which now represent a very serious public health problem (CitationCohen, 1992; CitationWalsh, 2000). In the food sector, the increasing prevalence of food pathogens in several food commodities is in large part due to a recent tendency to limit the use of traditional microbiological hurdles such as chemical additives and salt. For the safety of drug and food systems, the development of new antimicrobial agents is urgent. Over the past few decades, the search for new anti-microbial agents has occupied many research groups in the field of ethnopharmacology (CitationWallace, 2004). Much focus has been on determining the antimicrobial activity of plant extracts found in folk medicine (CitationRios & Recio, 2005). Several antimicrobial agents have been isolated from plants including secondary metabolites (such as xanthones (CitationNkengfack et al., 2002), coumarins (CitationOuahouo et al., 2004) and flavonoids (CitationKomguem et al., 2005)) and peptides (thionins (CitationFlorack & Stiekema, 1994), defensins (CitationBroekaert et al., 1995) and lectins (CitationWang & Ng, 1998)). Recently, ethanol extract from two Eremophila species containing antimicrobial compounds (terpenes and/or sterols) was used successfully to control the growth of L. monocytogenes in milk, and pâté as well as in Brie cheese (CitationOwen & Palombo, 2007).
In this study, the objective was to investigate the effect of antimicrobial compounds extracted from seeds of Oudneya africana R. Br. (Brassicaceae), Juniperus phoenicea L. (Cupressaceae), and Pistacia atlantica Desf. (Anacardiaceae), three wild plant species used for medicinal purposes in arid rural regions of Tunisia on food-borne pathogens. The plants were selected on the basis of ethnomedical application in the treatment of infections. The extracts were tested against common food-borne pathogens, including Escherichia coli, Pseudomonas aeruginosa, Listeria monocytogenes, Listeria ivanovii, and Listeria innocua. In addition, the physicochemical properties of active compounds were assessed, in an attempt to contribute to the use of these as alternative products for microbial control and food preservation.
Materials and methods
Selection and collection of plant materials
Three different plants, Juniperus phoenicea, Pistacia atlantica, and Oudneya africana, were collected from different localities in the region of Medenine, Tunisia in May 2001, May 2003, and April 2002, respectively. Voucher specimens are deposited in the Institute of Arid Regions of Tunisia Herbarium (Laboratoire d’écologie Pastorale) by Mohamed Neffati.
Preparation of plant extracts
All chemicals were of analytical grade. All extraction steps were done at 4°C. For each plant, 10 g of mature seeds were air-dried and extracted by the following three methods. Method 1 (CitationZhang & Lewis, 1997) comprised powdering the seeds with a mortar and pestle, stirring the powder in 0.05 M sulfuric acid (3 mL g−1) for 3 h, neutralizing the suspension with NaOH and removing the insoluble material by centrifugation at 10,000 g and subsequent microfiltration through a 0.22 μm membrane. For method 2 (CitationFujimura et al., 2003), the seeds were homogenized for 5 min with 100 mL of 0.05 M sodium acetate/acetic acid buffer (pH 4.8) in a Waring blender, stirred overnight and filtered through gauze and the filtrate was centrifuged at 10,000 g for 20 min. For method 3 (CitationSong et al., 2004), seeds were homogenized in 0.02 M phosphate buffer (pH 7.2) containing 0.1 M NaCl, stirred overnight, filtered through gauze, adjusted to pH 4 with acetic acid (50%, v/v), stirred for 4 h and then centrifuged at 10,000 g for 40 min. These aqueous extracts were further treated by precipitation with methanol or acetone followed by evaporation to the initial aqueous volume. The initial aqueous extracts as well as those mixed with methanol or acetone and their precipitates were tested for inhibitory activity against the food-borne pathogens listed below.
Characterization of the active compounds
The stability of the putative antimicrobial compounds under exposure to heat and organic solvents was evaluated for extract obtained by method 3 from the three plants. Extracts were thus boiled for 10, 20 or 30 min in a water bath or autoclaved (121°C) for 10 or 20 min. Solvent treatment consisted of mixing aqueous extracts with equal volumes of methanol, acetone, chloroform, hexane or acetonitrile for 2 h at room temperature, followed by evaporation to dryness and re-dissolving in 0.01 M phosphate buffer (pH 6). The residual activities of the extracts thus treated were measured by the critical dilution method as described below.
Assays for antimicrobial activity
Bacterial strains and media
Food-borne pathogens were provided by the Institut des Nutraceutiques et des Aliments Fonctionnels (INAF), Université Laval, Québec, Canada. Bacterial growth inhibition assays were performed using Listeria ivanovii (RBL30), Listeria innocua (RBL29), Listeria monocytogenes (LSD 530), Escherichia coli (ATCC 25922) and Pseudomonas aeruginosa (ATCC 15442). All strains were grown in tryptic soy broth (Difco Laboratories, Sparks, MD) supplemented with 0.6% (w/v) yeast extract (TSB) and incubated aerobically at 30°C. Cultures were transferred at least three times before use.
Agar diffusion method
The agar diffusion method described by (CitationTagg et al., 1976) was used. Tryptic soy agar with 0.6% yeast extract (Difco Laboratories, Sparks, MD) was autoclaved, cooled to 45°C, seeded at 1% (v/v) with an overnight culture of the indicator strain in TSB and poured into sterile Petri plates (25 mL each). Plates were then placed at 4°C for solidification. Wells were bored in the agar using the wide end of a sterile Pasteur pipette and 80 μL of plant extract was dispensed into each well. Before incubation, all plates were held at 4°C for 2 h. The plates were then incubated at 30°C for at least 24 h to develop inhibition zones and the diameter of these was measured.
Critical dilution method and MIC determination
The critical dilution method described by CitationTurcotte et al. (2004) was used. Briefly, a two-fold serial dilution of each extract in TSB was made in flat-bottomed 96-well polystyrene microplates (Microtest™, Becton Dickinson Labware, Franklin Lakes, NJ). Wells thus each contained 250 μL of dilution and were inoculated with 50 μL of overnight culture of the target bacteria diluted to a concentration of approximately 106 CFU/mL. Plates were incubated at 30°C for 16 h and absorbance at 650 nm was measured using a Thermo-max spectrophotometer (Molecular Devices, Sunnyvale, CA). The MIC was calculated from the highest dilution showing complete inhibition of the tested strain (OD equals OD of the blank). The MIC determinations were repeated independently three times. The results are presented as the median of three independent repetitions.
Inhibition of 24 h growth in liquid culture
Microplate wells containing TSB with plant extract in serial two-fold dilution were inoculated with bacterial strain at 1% (v/v) and incubated at 30°C for 24 h. Optical density at 650 nm was measured hourly.
Statistical analyses
Data were analyzed by ANOVA using a SAS system procedure (SAS Institute, Cary, NC). A multiple comparison test (LSD) was used to test the significant differences between the treatment means (P <0.05).
Results and discussion
Antimicrobial activity
The inhibitory activities of extracts of the three plants ( 5 mg/mL), as measured against the five pathogens by the agar diffusion test, are summarized in . The seeds of all three species of plant were thus found to contain antimicrobial agents, J. phoenicea appearing to be the most active. The three extraction methods showed variable effectiveness in extracting antimicrobial compounds, with method 3 being the most effective and yielding extract with a broad spectrum of activity. This method, which uses phosphate buffer, resulted in significant antibacterial activity against both Gram-positive and Gram-negative species, while the two other methods generated antimicrobial compounds active only against Gram-positive species. It was also noted that no precipitates showed any antimicrobial activity, while supernatants did. For all three methods, subsequent use of acetone or methanol resulted in a higher antimicrobial activity, especially for extract of J. phoenicea seeds. A potent substance also appears to have been concentrated by acetone in extract from O. africana.
Table 1. Antimicrobial activity of various extracts of Juniperus phoenicea, Pistacia atlantica and Oudneya africana as evaluated by the agar diffusion test.
Our work is the first to investigate the prevalence of antimicrobial compounds in plant species from the arid region of Tunisia. We have clearly shown that the spectrum of activity of these compounds depends a great deal on the extraction method used. In fact, while P. atlantica acid and acetate extracts were the most active against the test bacteria, only phosphate buffer extract was capable of inhibiting both Gram-positive and Gram-negative bacteria. For O. africana, the initial aqueous extracts of which showed no antibacterial effect, the acetone-soluble fraction produced strong inhibition of Listeria in the case of acetate extraction and inhibited all five species in the case of phosphate extraction. The noticeable feature of the phosphate buffer extracts is thus their antimicrobial activity over a broad range of food-borne pathogenic bacteria. The results obtained in the course of the present study are in agreement to a certain degree with the traditional uses of the plants investigated.
Characterization of the antimicrobial compounds
Extracts obtained by method 3 were selected for a further characterization. The growth of L. monocytogenes and E. coli incubated with different concentrations of extracts of the three plants are shown in . Dose-dependent inhibition curves were obtained and complete inhibition of both L. monocytogenes LSD 530 and E. coli ATCC 25922 was obtained with Juniperus and Pistacia extracts at a concentration of 12. 5 mg/mL. As evident from , the MIC values ranged from 1.56 to 3. 12 mg/mL for both J. phoenicea and P. atlantica extract, while O. africana had MICs values between 1.56 and 6. 25 mg/mL. Incubation of the phosphate extracts with various solvents for two hours did not affect activity. Active extracts of J. phoenicea and O. africana were completely insensitive to treatment with acetone, methanol, acetonitrile, chloroform, and hexane. However, Pistacia extract was sensitive to chloroform and hexane with loss of 50 and 25% of residual activity, respectively. It seems worthwhile to mention that all three extracts remained active after heating at 100°C for 30 min, and the Juniperus and Oudneya extracts (the latter being concentrated by acetone precipitation) were still active even after heating at 121°C for 20 min, with 96 and 70% of residual activity, respectively. In comparison, the extract of Pistacia was still active after 10 min of treatment at 121°C (70% of residual activity) but not 20 min (no antimicrobial activity). Heat stability is a widely sought criterion for the selection of bioactive compounds. Our results have shown that the active principles in the plant extracts obtained in this study were quite resistant to heating, remaining at least partially active even after 20 min at 121°C. This heat stability would be a very useful characteristic, especially in the case of antimicrobial compounds to be used as food preservatives, since many food-processing procedures involve a heating step.
Figure 1. Growth of Listeria monocytogenes (A, C, E) and Escherichia coli (B, D, F) in the presence of extracts (by method 3) of seeds of (A, B) Juniperus phoenicea, (C, D) Pistacia atlantica and (E, F) Oudneya africana (acetone-soluble portion) in tryptic soy broth. Concentrations (mg/mL) of extract were 12.5 (triangle), 6.25 (square), 3.125 (diamond) and 0 (circle).
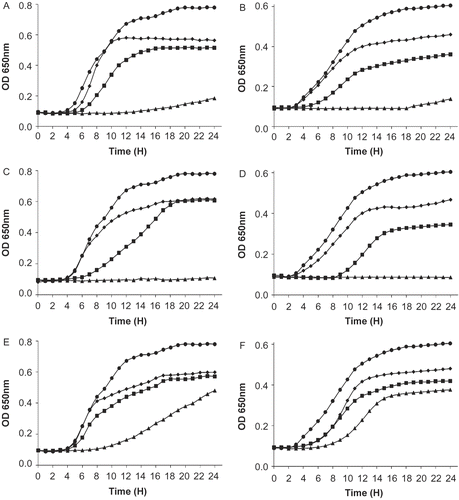
Table 2. Determination of MIC of selected plant extracts against food-borne pathogens.
Phytochemical and further pharmacological studies are important tasks for the future in order to better understand the effects of these important pharmaceutical resources. In the present work, we have clearly demonstrated the potential of plants recovered from arid regions of Tunisia as a source of antimicrobial agents. We have also shown that some of these compounds have a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria. The results of this study are quite encouraging; these compounds may offer a promising alternative for purposes of food preservation. Further studies in progress aim to examine the mode of action of these compounds within the scope of challenge tests in foodstuffs.
Acknowledgement
This research was supported by the Ministry of Higher Education, Scientific Research and Technology, Republic of Tunisia.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Broekaert WF, Terras FR, Cammue BP, Osborn RW (1995): Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol 108: 1353–1358.
- Cohen ML (1992): Epidemiology of drug resistance: Implications for a post-antimicrobial era. Science 257: 1050–1055.
- Florack DE, Stiekema WJ (1994): Thionins: Properties, possible biological roles and mechanisms of action. Plant Mol Biol 26: 25–37.
- Fujimura M, Minami Y, Watanabe K, Tadera K (2003): Purification, characterization, and sequencing of a novel type of antimicrobial peptides, Fa-AMP1 and Fa-AMP2, from seeds of buckwheat (Fagopyrum esculentum Moench.). Biosci Biotechnol Biochem 67: 1636–1642.
- Komguem J, Meli AL, Manfouo RN, Lontsi D, Ngounou FN, Kuete V, Kamdem HW, Tane P, Ngadjui BT, Sondengam BL, Connolly JD (2005): Xanthones from Garcinia smeathmannii (Oliver) and their antimicrobial activity. Phytochemistry 66: 1713–1717.
- Nkengfack AE, Mkounga P, Meyer M, Fomum ZT, Bodo B (2002): Globulixanthones C, D and E: three prenylated xanthones with antimicrobial properties from the root bark of Symphonia globulifera. Phytochemistry 61: 181–187.
- Ouahouo BM, Azebaze AG, Meyer M, Bodo B, Fomum ZT, Nkengfack AE (2004): Cytotoxic and antimicrobial coumarins from Mammea africana. Ann Trop Med Parasitol 98: 733–739.
- Owen RJ, Palombo EA (2007): Anti-listerial activity of ethanolic extracts of medicinal plants, Eremophila alternifolia and Eremophila duttonii, in food homogenates and milk. Food Control 18: 387–390.
- Rios JL, Recio MC (2005): Medicinal plants and antimicrobial activity. J Ethnopharmacol 100: 80–84.
- Song X, Zhou Z, Wang J, Wu F, Gong W (2004): Purification, characterization and preliminary crystallographic studies of a novel plant defensin from Pachyrrhizus erosus seeds. Acta Crystallogr D Biol Crystallogr 60: 1121–1124.
- Tagg JR, Dajani AS, Wannamaker LW (1976): Bacteriocins of Gram-positive bacteria. Bacteriol Rev 40: 722–756.
- Turcotte C, Lacroix C, Kheadr E, Grignon L, Fliss I (2004): A rapid turbidometric microplate bioassay for accurate quantification of lactic acid bacteria bacteriocins. Int J Food Microbiol 90: 283–293.
- Wallace RJ (2004): Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc 63: 621–629.
- Walsh C (2000): Molecular mechanisms that confer antibacterial drug resistance. Nature 406: 775–781.
- Wang H, Ng TB (1998): Ribosome inactivating protein and lectin from bitter melon (Momordica charantia) seeds: Sequence comparison with related proteins. Biochem Biophys Res Commun 253: 143–146.
- Zhang Y, Lewis K (1997): Fabatins: New antimicrobial plant peptides. FEMS Microbiol Lett 149: 59–64.
