Abstract
This study was undertaken to determine the cardioprotective effect of hydroxysafflor yellow A (HSYA), at doses of 2, 4 or 8 mg/kg, extracted from the flower of the safflower plant, Carthamus tinctorius L. (Asteraceae), on acute myocardial ischemia induced by left anterior descending coronary artery (LAD) ligation in rats. The myocardial infarction size (MIS), creatine kinase (CK-MB) activity, endothelial nitric oxide synthase (eNOS) activity, superoxide dismutase (SOD) activity, malondialdehyde (MDA) content and nitric oxide (NO) content were measured. The results showed that administration with hydroxysafflor yellow A at doses of 4 or 8 mg/kg resulted in a reduction in myocardial infarction size. Hydroxysafflor yellow A (4 or 8 mg/kg) inhibited not only the elevation of creatine kinase activity and malondialdehyde content but also the reduction of superoxide dismutase activity, endothelial nitric oxide synthase activity and nitric oxide content. The findings suggested that hydroxysafflor yellow A (4 or 8 mg/kg) exerted cardioprotective effects against acute myocardial ischemic injury by regulating superoxide dismutase activity and endothelial nitric oxide synthase activity.
Introduction
Ischemic heart diseases, especially acute myocardial ischemia, remain the leading cause of death in both developed and developing countries over the past quarter century. Reduction of mortality rate and prevention of myocardial infarction are of utmost importance. Reactive oxygen species, which possess highly reactive and toxic properties, can be generated as a result of ischemia and exacerbate the degree of myocardial damage sustained by the ischemic myocardium (CitationWattanapitayakul & Bauer, 2001). As a consequence, animals have developed an effective defense system to cope with unwanted and toxic oxygen species. In the heart, defense mechanisms include enzymes such as SOD, catalase, and glutathione peroxidase, plus other endogenous antioxidants. In pathological or disease conditions, such as myocardial infarction, stroke and others, the production of free radicals may override the scavenging effects of antioxidants leading to oxidative stress (CitationZhu et al., 2004). In recent years, Chinese medicinal herbs and their extracts have been given more attention for prevention and treatment of ischemic heart diseases. Carthamus tinctorius L. (Asteraceae) is listed in the pharmacopoeia of China and has an indication including ameliorating circulation and removing blood stasis. Safflor yellow is aqueous extracts from C. tinctorius and contains HSYA, safflor yellow B, safflomin A, and safflomin C (CitationRomano et al., 1993). The main chemical component of the safflower yellow pigments is HSYA. CitationJin et al. (2004a) reported that HSYA had an antioxidative effect in vitro. Recent study showed that HSYA reduced brain injury caused by focal cerebral ischemia/reperfusion by means of attenuating the elevation of MDA content and the decrease of SOD activity (CitationWei et al., 2005). Based on these reports, we hypothesis that HSYA may exert cardioprotective effects on acute myocardial ischemia through reducing oxidant stress. In this study we investigated the experimental therapeutic effects and mechanisms of HSYA on acute myocardial ischemia damage by a left anterior descending coronary artery ligation model in rats.
Materials and methods
Samples of C. tinctorius, collected from Qingyuan country of Yunnan Province during June 2005, were kindly supplied by Shandong Luye Pharmaceutical. Its identity was confirmed by Prof. Li with authentic specimens kept at the herbarium, Yantai Univeristy. A voucher specimen was deposited in the Department of Medicinal Chemistry, Yantai Univeristy. HSYA was prepared in accordance with the method of CitationJin et al. (2004b). C. tinctorius flower (2 kg) were cut into small pieces, pounded into powder and then extracted twice with distilled water (40 L) for 24 h each time at room temperature. After filtering, the aqueous extracts were added to a non-polar macroporous resin column (DM-130, Anhui, China) and impurities such as sugar were washed out by water. Then the HSYA was eluted by 10% ethanol. The eluent was freeze-dried, finally giving a yellow amorphous powder. The yield was 0.4% (w/w). Further analysis by High Performance Liquid Chromatography (HPLC) showed that the content of HSYA in the resultant extract was 98%. High-resolution electrospray tandem mass spectrometry (ES-MS) exhibited an [M –H]− ion peak at m/z = 611.1614 and the molecular formula was determined to be C27H32O16 (). Acetonitrile and methanol were HPLC grade and all other chemicals were of analytical grade. Distilled water, prepared from demineralized water, was used throughout the study.
Experimental animals
The experiments were performed according to the guidelines established by the Guide for the Care and Use of Laboratory Animals of Yantai Univeristy, and were approved by the local Ethics Committee. Male Sprague-Dawley rats weighing 260-280 g were obtained from the Experimental Animal Center of Shandong Province (Jinan, China). All the rats were housed in diurnal lighting conditions (12 h/12 h) and allowed free access to food and water.
Chemicals and reagents
Thiobarbituric acid, nitroblue tetrazolium and triphenyltetrazolium chloride were purchased from Sigma Chemical Co. (St. Louis, MO). The reagents for measuring serum creatine kinase activity and eNOS activity were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). NO detection kit was from Eastern Asia Radioimmunity Research Institute (Beijing, China).
Induction of acute myocardial ischemia in rats
Ninety-two SD rats were randomly divided into 5 groups. The rats in HSYA groups were tested with HSYA at doses of 2, 4, or 8 mg/kg via the tail vein once a day for 3 days continually. An acute myocardial infarction model was produced by ligation of the left coronary artery as described previously (CitationStanton et al., 2000) with minor modification. Briefly, 30 min after the last dose, rats were anesthetized with chloralose (300 mg/kg intraperitoneally). Endotracheal intubation was performed under direct visualization. The rats were ventilated with air (tidal volume, 3 mL/100 g; respiratory rate, 60 times/min) by a ventilator (DW-3000, Anhui, China). A thoracotomy was performed in the fourth intercostal space under sterile conditions. A 1.0 silk thread was passed around the LAD near its origin using a tapered needle. Both ends of the thread were passed through a 1 cm long polyethylene tube (outer diameter, 2 mm), which was used to ligate the coronary artery by pulling the thread. Then the thoracic cavity was sutured. Body temperature was measured by an electric thermometer placed in the rectum and maintained at 37°C by a heating pad placed under the rats. Sham-operated rats were subjected to the same procedures without LAD ligation.
Measurement of MIS
At 6 h post-occlusion, the heart was harvested and the ventricle of heart was cut from the apex to the base into four transverse slices and incubated in 1% (w/v) triphenyltetrazolium chloride for 10 min. By this staining, normal myocardium appeared brick red and the infarct area was unstained. The slices were immediately fixed with 10% formalin for 24 h. The ischemic zone was dissected from the non-ischemic zone by the same lab assistant who was blind to the treatment. MIS was calculated as: weight of ischemic zone/total weight of the ventricle × 100% (CitationNi et al., 2001; CitationHaiyun et al., 2004).
Measurement of CK-MB activity
The blood was sampled from the abdominal aorta at 6 h post-occlusion and left at room temperature for 1 h to allow complete clotting. After centrifugation at 1500 g for 15 min, serum was separated from blood cells. Serum CK-MB activity was measured using a commercial kit according to the instructions provided by the manufacturer.
Measurement of NOS, NO, SOD and MDA
Six heart tissues in the sham and eight heart tissues in the other groups were excised, rinsed in ice-cold isotonic saline, and then homogenized in 0.1 M ice-cold phosphate buffer (pH 7.4; 1:10 w/v) for biochemical assays (CitationYu et al., 2007). The total protein was estimated by the method of CitationLowry et al. (1951). eNOS activity was measured with a commercial kit according to the instructions provided by the manufacturer. Because most of the NO is rapidly converted to nitrite and further to nitrate, the levels of nitrite/nitrate were measured with the NO detection kit according to the manufacturer’s instructions. Briefly, nitrate was converted to nitrite with Aspergillus nitrite reductase, and the total nitrite was measured with the Griess reagent. The absorbance was determined at 540 nm with a microplate reader (Bio-TEK,Windoski, VT). SOD activity was determined by the NBT reduction method (CitationMcCord & Fridovich, 1969). The determination of MDA content in heart tissues was performed according to the method of CitationOhkawa et al. (1979).
Statistical analysis
All data are expressed as the means ± standard deviation (SD). Differences among groups were analyzed by one-way ANOVA, then Student-Newman-Keuls test. Values of p <0.05 were considered statistically significant.
Results
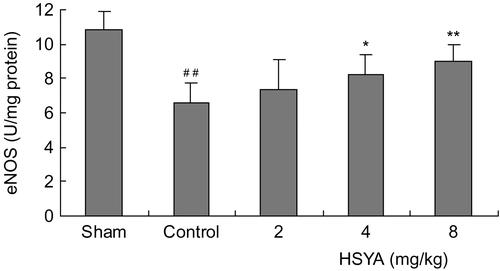
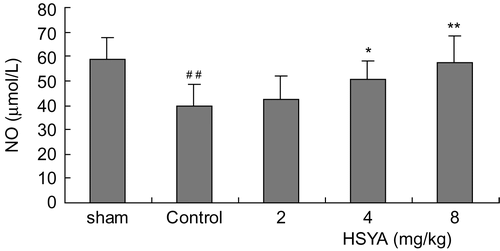
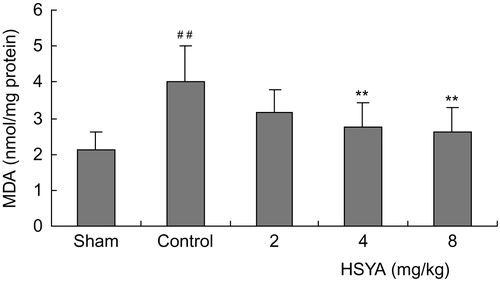
The survival rates at the end of the observation of the rats were 100% (12/12) in the sham group, 70% (14/20) in control group, 75% (15/20) in the HSYA (2 mg/kg) group, 75% (15/20) in the HSYA (4 mg/kg) group and 80% (16/20) in the HSYA (8 mg/kg) group, respectively. There was no significant difference between the HSYA treatment group and the control group in survival rates. The results showed that treatment with HSYA at doses of 4 or 8 mg/kg significantly reduced MIS induced by LAD ligation. LAD ligation resulted in a significant increase of serum CK-MB activity. Administration with HSYA at doses of 4 or 8 mg/kg led to a reduction in the increased CK-MB activity (). SOD activity was significantly lower, while MDA content was significantly higher in the control group than that in the sham group (p <0.01). Treatment with HSYA at doses of 4 or 8 mg/kg attenuated the decrease of SOD activity and the increase of MDA content (p <0.01) ( and ). As shown in and , eNOS activity and NO content in heart tissues showed a significant decrease in the control group (p <0.01). The decrease of eNOS activity and NO content in the group treated with HSYA at doses of 4 or 8 mg/kg were inhibited when compared with that in the control group (p <0.05, p <0.01).
Table 1. Effect of HSYA on myocardial infarction size (MIS) and creatine kinase (CK-MB) activity in acute myocardial ischemic rats.
Figure 2. Effect of HSYA on SOD activity in heart tissues of acute myocardial ischemic rats. Data are means ± SD of 8 rats, except 6 rats in sham group. ##p <0.01 versus sham group; *p <0.05, **p <0.01 versus control group.
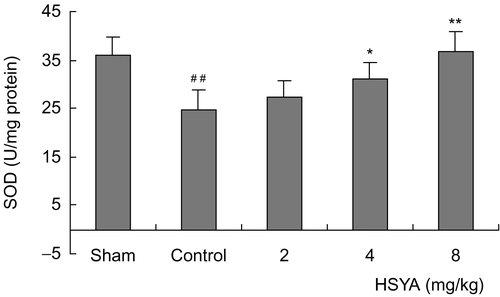
Figure 3. Effect of HSYA on MDA content in heart tissues of acute myocardial ischemic rats. Data are means ± SD of 8 rats, except 6 rats in sham group. ##p <0.01 versus sham group; **p <0.01 versus control group.
Discussion
It is well known that coronary artery occlusion plays a key role in acute myocardial infarction. To investigate the cardioprotective action of HSYA, we employed a typical acute myocardial infarction model induced by LAD ligation. Our results showed that HSYA had obvious cardioprotective effects and could significantly reduce myocardial infarction size induced by LAD ligation. LAD ligation leads to myocardial ischemia and myocardial necrosis. CK-MB, a cardiac-specific marker of acute myocardial infarction or an indicator for myocardial tissue injury, would leak into serum from cells. HSYA administration restrained the increase of CK-MB activity, which confirmed the protective effects of HSYA against myocardial ischemic damage.
Oxidative stress is one of the major concerns in the treatment of ischemic heart diseases. Some studies have reported that acute myocardial ischemia generated numerous free radicals, causing damage to lipids and DNA (CitationBolli et al., 1988: CitationFreeman & Crapo, 1982). Accordingly, the organism protects itself from oxidative stress by endogenous free radical scavenger systems. One of the endogenous antioxidant enzymes is SOD which can scavenge superoxide radicals. This enzyme contributes to maintaining physiological levels of oxygen and hydrogen peroxide by hastening the dismutation of oxygen radicals and eliminating organic peroxides and hydroperoxides. In our study, HSYA appears to work by preserving SOD activity as well as inhibiting lipid peroxidation in ischemic myocardium, as shown by the depressed formation of MDA content. This implies that the cardioprotective effects of HSYA contribute to saving endogenous SOD activity under oxidative stress, at least in part. However, we cannot be certain whether HSYA exerts such effects by stimulating the expression of endogenous SOD at the mRNA or protein levels and then enhances SOD function subsequently.
The endothelial NO synthase (eNOS) is expressed in the endothelium, where it produces NO from l-arginine. NO diffuses from the endothelium to the vascular smooth muscle cells, where it increases the concentration of GMP by stimulating soluble guanylate cyclase, leading to vascular relaxation. Basal release of NO by the endothelium contributes to basal vascular tone and regulates blood flow and blood pressure. NO also inhibits the proliferation of smooth muscle cells. Furthermore, NO protects against platelet aggregation in vivo and in vitro and inhibits platelet adhesion to vascular endothelium. In addition, NO inhibits leukocyte adhesion to endothelium. It was reported that short ischemic durations of 30 min or less did not affect eNOS activity. However, with longer ischemic durations of 60 min or more a marked loss of eNOS activity was observed (CitationGiraldez et al., 1997) and then myocardial ischemia was aggravated. In our experiment, HSYA attenuated the reduction of eNOS activity induced by acute myocardial ischemia and thus enhanced NO content. Considering that NO can limit infarct size, maintain endothelial function, and improve myocardial contractility (CitationPernow et al., 1994; CitationLefer et al., 1993), it is possible that the cardioprotective effects of HSYA will contribute to saving eNOS activity and maintain NO bioavailability during myocardial ischemia.
In conclusion, the present experiment showed that HSYA exerted cardioprotective effects against acute myocardial ischemia injury in rats, and indicated HSYA as an effective and promising medicine for treatment of ischemic heart disease.
Acknowledgements
This study was supported by the National High Technology Research and Development Program of China (No. 2002AA283139), the Young Scientist Foundation of Yantai University, and Shandong Luye Pharmaceutical Limited Company.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB (1988): Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl-N-tert-butyl nitrone. J Clin Invest 82: 476–485.
- Freeman BA, Crapo JD (1982): Biology of disease: Free radicals and tissue injury. Lab Invest 47: 412–426.
- Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL (1997): Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. J Biol Chem 34: 21420–21426.
- Haiyun L, Yijia L, Honggang L, Honghai W (2004): Protective effect of total flavones from Elsholtzia blanda (TFEB) on myocardial ischemia induced by coronary occlusion in canines. J Ethnopharmacol 94: 101–107.
- Jin M, Li JR, Wu W (2004a): Antioxidative effect of hydroxysafflor yellow A. Chin Tradit Herb Drugs 35: 665–666 [article in Chinese].
- Jin M, Gao ZC, Li JR, Zang BX, Wu W (2004b): Preparation of safflor yellow and hydroxysafflor yellow A by macroporous resin column chromatography. Chin Tradit Herb Drugs 35: 25–28 [article in Chinese].
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Lefer DJ, Nakanishi K, Johnston WE, Vinten-Johansen J (1993): Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion in dogs. Circulation 88: 2337–2350.
- McCord JM, Fridovich I (1969): Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049–6055.
- Ni Y, Pislaru C, Bosmans H, Pislaru S, Miao Y, Bogaert J, Dymarkowski S, Yu J, Semmler W, Van de, Werf F, Baert AL, Marchal G (2001): Intracoronary delivery of Gd-DTPA and gadophrin-2 for determination of myocardial viability with MR imaging. Eur Radiol 11: 876–883.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Pernow J, Uriuda Y, Wang QD, Li XS, Nordlander R, Rydeen L (1994): The protective effect of l-arginine on myocardial injury and endothelial function following ischaemia and reperfusion in the pig. Eur Heart J 15: 1712–1719.
- Romano C, Price M, Bai HY, Olney JW (1993): Neuroprotectants in Honghua: Glucose attenuates retinal ischemic damage. Invest Ophthalmol Vis Sci 34: 72–80.
- Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT (2000): Altered patterns of gene expression in response to myocardial infarction. Circ Res 86: 939–945.
- Wei X, Liu H, Sun X, Fu F, Zhang X, Wang J, An J, Ding H (2005): Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett 386: 58–62.
- Wattanapitayakul SK, Bauer JA (2001): Oxidative pathways in cardiovascular disease: Roles, mechanisms, and therapeutic implications. Pharmacol Ther 89: 187–206.
- Yu C, Fu F, Yu X, Han B, Zhu M (2007): Cardioprotective effect of ocotillol, a derivate of pseudoginsenoside F11, on myocardial injury induced by isoproterenol in rats. Arzneimittelforschung 57: 568–572.
- Zhu YZ, Huang SH, Tan BK, Sun J, Whiteman M, Zhu YC (2004): Antioxidants in Chinese herbal medicines: A biochemical perspective. Nat Prod Rep 21: 478–489.