Abstract
The present work describes the phytochemical investigation of the aerial parts of Amanoa almerindae Leal (Euphorbiaceae). After fractionation of the organic extracts, four pentacyclic triterpenes (friedelin, lupeol, betulin, betulinic acid), three steroids (β-sitosterol, sitostenone, daucosterol), a lignan (49-demethyl- deoxypodophyllotoxin) and for first time in this genus the presence of the biflavones amentoflavone, sequoiaflavone, and putraflavone were identified. The structures of isolated compounds were established by spectroscopic methods and confirmed by comparison with reference samples and literature data. No previous phytochemical study of Amanoa almerindae has been reported, and to the best of our knowledge, this is the first report of biflavonoids in the Amanoa genus.
Introduction
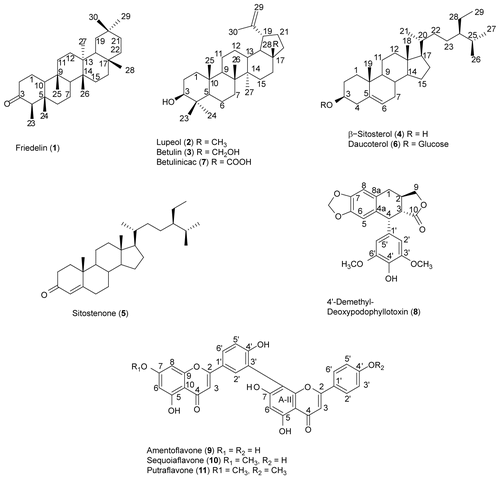
The genus Amanoa belongs to subfamily Phyllanthoideae in the Euphorbiaceae, and comprises about 15 species, which are spread in tropical regions of America and Africa (CitationPittier et al., 1947). In Venezuela, six are present in the Guayana and Amazonas regions. Amanoa almerindae Leal, known by the vernacular name “reventillo”, is a medium-sized tree that is widespread in the Venezuelan Amazonas, and in the Punos region in Perú (CitationSteyermark et al., 1999). According to our search, this is the second report on the chemical composition of Amanoa species. The literature shows few studies of the genus Amanoa. The most known studies are from Amanoa oblongifolia Muell., which report the isolation of terpenes and lignans (CitationFang et al., 1985; CitationNanayakkara et al., 1986; CitationMacRae et al., 1988) and its antiviral activity (CitationJassim & Naji, 2003). In the continuation of our investigation on phytochemical and pharmacological evaluation of the Euphorbiaceae of Venezuela (CitationSuarez et al., 2003, Citation2004, Citation2005, Citation2006; CitationCanelon et al. 2005) we chose the species Amanoa almerindae, which was collected on the borders of the Sipapo River, Amazonas state, Venezuela. The aim of the present paper is to report the first phytochemical investigation on the aerial parts of this plant, which led to the isolation and structural elucidation of four triterpenes, 1, 2, 3, 7, the steroids, 4, 5, 6, the lignan 8 and, for first time in this genus, the presence of the biflavones amentoflavone (9), sequoiaflavone (10), and putraflavone (11), see . The structures of the isolated compounds were established by spectroscopic analysis, mainly 1H and 13C using extensive 2D experiments and comparison with literature data. Pharmacological evaluation of Amanoa almerindae, which is underway in our lab, shows that the aqueous extract presented interesting antinociceptive activity.
Materials and methods
General
NMR spectra were recorded on a Bruker AMX-500 spectrometer operating at 500 MHz (1H) and 125 MHz (13C), respectively. 1H and 13C chemical shifts (δ, ppm) are relative to residual solvent signals. MS were determined on a Varian Saturn-2000 instrument. IR spectra were recorded on a Perkin Elmer 1600 Series FTIR spectrometer. UV spectra were measured on a Shimadzu UV-3101 spectrometer. Thin layer chromatograms were run on aluminum sheets precoated with silica gel 60 F254 (20 × 20 cm, 0. 2 mm thick) or with RP-18 F254 from Merck. The visualization of the TLC plates was carried out with a UV lamp (254 and 366 nm) before spraying the plates with p-anisaldehyde in H2SO4.
Plant material
The aerial parts of Amanoa almerindae, were collected on the borders of the Sipapo River, Amazonas state, Venezuela, in May 2000. Taxonomic identification was made by Dr. Anibal Castillo. A voucher specimen (AC 8989) has been deposited in the Herbarium Victor Manuel Ovalles (MYF) of the Faculty of Pharmacy, Universidad Central de Venezuela.
Extraction and isolation
The air-dried powdered leaves (100 g) were extracted by Soxhlet with methanol. After evaporation of the methanol (25.5 g), water was added and partitioned with hexane and with dichloromethane. Evaporation of the solvents at reduced pressure furnished 4.5 g of hexane extract, 2.4 g of dichloromethane extract and the residual methanol extract was 18.7 g. The stems (170 g) were extracted successively in a Soxhlet first with hexane, then with dichloromethane, and finally with ethyl acetate to give, respectively, 0.53, 0.58, and 0.77 g. The bulk of each extract was chromatographed separately over Si-gel using hexane and increasing amounts of dichloromethane, EtOAc and then methanol. From the hexane extract obtained from leaves, 60 fractions (ca. 25 mL each) were collected, which were combined on the basis of their profiles on TLC to give: friedelin (1) (27.2 mg) Citation(Gottlieb et al., 1985), lupeol (2) (11.2 mg) (CitationSchulichin et al., 1980), betulin (3) (12.8 mg) (CitationTinto et al., 1992), and β-sitosterol (4) (32.5 mg) (CitationDe Pascual et al., 1987; CitationSatyanarayana et al., 1992). From the hexane extract of the stems, the compounds isolated were: 1, 3, and β-sitostenone (5) (17.8 mg) (CitationDella-Greca et al., 1990). The dichloromethane extract obtained from the leaves provided, after column chromatography with Si-gel using dichloromethane-EtOAc and MeOH in mixtures of increasing polarity, the compounds daucosterol (6) (23.6 mg) (CitationKojima et al., 1990) and betulinic acid (7) (34.2 mg) (CitationSchulichin et al., 1980). The methanol extract obtained from the leaves was subjected to column chromatography using C18 reverse-phase column chromatography, eluted with water-acetone (80:20 -70-30) to give 49-demethyl-deoxypodophyllotoxin (8) (56.3 mg) (CitationHudson et al., 1989) and the biflavones: amentoflavone (9) (25.7 mg) (CitationChaabi et al., 2007), sequoiaflavone (10) (18.2 mg) (CitationLi et al., 2003), and putraflavone (11) (15.9 mg) (CitationSuàrez et al., 2003). The lignan 8 and the flavonoid 11 were also obtained from the EtOAc extract of the stems.
Results and discussion
The 1H NMR and 13C NMR spectra were used to assign the signals corresponding to methyl, methylene, methine, and quaternary carbons in the structural elucidation of the steroids and triterpene compounds (1–7). Comparison with authentic substances and literature data confirmed the well established structure of each of these metabolites, isolated from leaves and stems of Amanoa almerindae.
The 1H, 13C NMR, HMQC, and HMBC experiments were used to confirm the occurrence of 49-demethyl-deoxopodophyllotoxin (8). All the data obtained were consistent with the NMR data reported for this lignan, which was previously isolated from Amanoa oblongifolius (CitationFang et al., 1985). The presence of this lignan among the steroids and triterpenes previously described could be considered as a valuable chemotaxonomic marker for the genus.
To identify completely each one of the biflavones, amentoflavone (9), sequoiaflavone (10), and putraflavone (11), a series of UV and NMR experiments provided strong supporting evidence for each of the proposed structures (CitationAgrawal, 1989), which were corroborated with mass spectrometry and 13C NMR analysis (). The first report of biflavonoids in species of Amanoa, presented by us in this communication, may be useful for future chemical profiles of the genus. The occurrence of the biflavonoids could be considered as valuable chemotaxonomic markers for the genus, and this whole work strongly supports the placement of Amanoa within the spurge family, as triterpenes, steroids and biflavonoids are common metabolites in many of its species.
Table 1. 13C NMR data of Amanoa almerindae biflavones.
Acknowledgement
This work was supported in part by a Grant for FONACIT (G200500389) and by CDCH of the Universidad Central de Venezuela Providence No. 06-017-2007 to A.I. Suàrez.
References
- Agrawal PK (1989). Carbon-13 NMR of Flavonoids. Amsterdam: Ed. Elsevier, pp. 283–354.
- Canelón D, Compagnone RS, Castillo A, Suárez AI (2005): Chemical constituents of Senefelderopsis chiribiquetensis. Biochem Sys Eco 33:1303–306.
- Chaabi M, Antheaume C, Weniger B, Justininiano H, Lugnier C, Lobstein A (2007): Biflavones of Decussocarpus rospigliossi as phosphodiesterase inhibitors. Planta Med 73:1284–1286.
- Della-Greca M, Monaco P, Previtera L (1990): Stigmasterols from Typha latifolia. J Nat Prod 5:1430–1435.
- De Pascual TJ, Urones JG, Marcos IS, Basame P, Sexmero-Cuadrado MJ, Fernández-Moro R (1987): Triterpenes from Euphorbia broteri. Phytochemistry 26:1767–1776.
- Fang X, Nanayakkara NPD, Phoebe CHH, Pezzuto JM, Kinghorn AD, Farnsworth NR (1985): Plant anticancer agents XXXVII. Constituents of Amanoa oblongifolia. Planta Med 51:346–347.
- Gottlieb H, Ramaiah P, Lavie D (1985): 13C NMR Signal assigment of friedelin and 3α-hydroxyfriedelan-2-one. Magn Reson Chem 23:616–620.
- Hudson JB, MacRa WD, Tower GH (1989): The antiviral action of lignans. Planta Med 55: 531–535.
- Jassim SAA, Naji MA (2003): Novel antiviral agents: A medicinal plant perspective. J Appl Microb 95:412–427.
- Kojima H, Sato N, Hatano A, Ogura H (1990): Sterol glucosides from Prunella vulgaris. Phytochemistry 29:2351–2355.
- Li S-H, Zhang H-J, Niu X-M, Yao P, Sun H-D, Fong HHS (2003): Chemical constituents from Amentotaxus yunnanensis and Torreya yunnanensis. J Nat Prod 66:1002–1005.
- MacRae WD, Hudson JD, Towers GH (1988): Alpha-(-)-peltatin, an antiviral constituent of Amanoa aff. oblongifolia. J Ethnopharmacol 22:223–236.
- Nanayakkara DNP, Kinghorn AD, Farnsworth NR (1986): New hydroxylated eudesmane sesquiterpenoids from Amanoa oblongifolia. J Chem Res 12:454–455
- Pittier H, Lasser T, Schee L, Luces Z, Badillo V (1947): Catalogo de la Flora Venezolana. Litografia y Tipografia Vargas, Caracas. Vol. II, p. 65.
- Satynarayana V, Krupadanam GLD, Srimannarayana G (1992): Tetracyclic triterpenes from the latex of Euphorbia nivula. Fitoterapia 63:82–83.
- Shulichin M, Yamasaki K, Kasai R, Tanaka O (1980): 13C Nuclear magnetic resonance of lupane-type triterpenes lupeol, betulin and betulinic acid. Chem Pharm Bull 28:1006–1008.
- Steyermark, JA, Berry PE, Yatskievych K, Host BK (1999): Flora of the Venezuelan Guayana, Vol 5. Missouri Botanical Garden Press, St Louis, MO, USA, pp. 95–99.
- Suarez AI, Diaz B, Delle Monache F, Compagnone RS (2003): Biflavonoids from Podocalyx loranthoides. Fitoterapia 74:473–475.
- Suarez AI, Blanco Z, Delle Monache F, Compagnone RS (2004): Three new glutarimide alkaloids from Croton cuneatus. Nat Prod Res 18:421–426.
- Suarez AI, Tapias E, Compagnone RS, Tillett S, Diaz B, Canelòn D, Blanco Z (2005): Chemical constituents from Croton huberi. Rev Fac Farm 68:14–19.
- Suarez AI, Blanco Z, Compagnone RS, Salazar-Bookaman MM, Zapata V, Alvarado C (2006): Anti-inflammatory activity of Croton cuneatus aqueous extract. J Ethnopharmacol 105: 99–101.
- Tinto WF, Blair LC, Alli A, Reynolds WF, McLean S (1992): Lupane triterpenoids of Salacia cordata. J Nat Prod 55:395–398.