Abstract
This research aimed to study the effects of Myristica fragrans Houtt. (Myristicacae) oil on male reproductive functions in Wistar strain rats, the oil of Myristica fragrans at the dose levels of 0.2, 0.4, and 0.5 mL/kg body weight/day for 60 days. The oil reduced fertility to 0% by 60 days of treatment at the highest dose level. The results also revealed the oil caused significant reduction in the weight of testis, epididymis, seminal vesicle, ventral prostate and vas deferens. Sperm motility as well as sperm density were significantly reduced which resulted in the reduction of male fertility by 100% at the highest dose level tested. Leydig cell nuclear area and mature Leydig cell numbers were significantly reduced when compared with controls. The round spermatids were decreased by 72.71%. The hematological parameters show a non-significant change when compared with the control group. A recovery of 80% fertility was recorded after withdrawal of 120 days, proving that the drug is safe to use. These results suggest the antifertility and recovery effect from the oil of Myristica fragrans in male albino rats.
Introduction
Plants that were considered of no value are now being investigated, evaluated, and developed into drugs with little or no side effects. Fertility regulation with plant preparations has been reported in ancient literature of indigenous systems of medicine. Myristica fragrans Houtt. (Myristicacae) is a tropical, dioecious evergreen tree native to the Molluccas, of the Spice Islands of Indonesia. Its seeds were reported to be useful in various ailments (CitationChopra et al., 1956). Dried kernels and oil of Myristica fragrans are used as aromatic, spasmolytic, and anxiogenic agents (CitationSonavane et al., 2002).
Pharmacologically and therapeutically, the value of Myristica fragrans seed oil is for the treatment of diarrhea and controlling blood pressure (CitationGrover et al., 2002). The effect of the oil of nutmeg on the fertility and induction of meiotic chromosome rearrangements in mice and their first generation have been studied (CitationPecevski et al., 1981).
Since no attention has been drawn towards contraceptive effects, and the mode of action of Myrsitica fragrans has not been reported in detail, the present communication was undertaken to study the possible antifertility effects of Myristica fragrans oil at 0.5 mL/kg body weight (bw) for 60 days in male albino rats, and also to interpret the recovery after 120 days of withdrawal of the treatment.
Materials and methods
Plant material
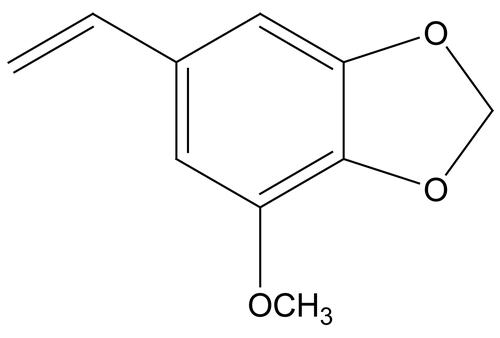
The oil of Myristica fragrans was purchased from the Himalaya Drug Company, Banglore, India. Myristicin is the chief constituent previously isolated from the oil ().
Animals used
Thirty adult male Wistar strain rats weighing 180–200 g were used. They were maintained at standard conditions (12 h light/12 h night, 26° ± 2°C) in wire-bottomed polypropylene cages with free access to standard rat pellet diet (Aashirwad, Chandigarh) and provided water ad libitum. All the described experiments have been carried out under the approval of the departmental ethics committee. The Guidelines for the Care and Use of Animals for scientific Research (CitationINSA, 2000) were strictly followed throughout the course of investigation.
Before starting the experiments, the male rats were kept for fertility tests, the number and size of litters were recorded.
Study protocol
The rats were divided into three groups containing ten animals in each.
Group – I (Control) Control or vehicle treated (1.0 mL olive oil)
Group – II (Treatment) 0.2 mL Myristica fragrans oil/kg bw/day for 60 days
Group – III (Treatment) 0.4 mL Myristica fragrans oil/kg bw/day for 60 days
Group – IV (Treatment) 0.5 mL Myristica fragrans oil/kg bw/day for 60 days
Group – V (Recovery) 0.5 mL Myristica fragrans oil/kg bw/day for 60 days and kept for 120 days for recovery.
Myristica fragrans oil was diluted in 0.5 mL olive oil and administered orally; the animals were weighed before and after the treatment.
Fertility test
The mating exposure tests of control and treated groups were performed on day 55 using the method of CitationWHO (1983). After mating the males were removed for autopsy.
The animals were weighed and autopsied under light ether anesthesia 24 h after the last dose of the treatment. Blood samples were collected by cardiac puncture in heparinized tubes for hematological studies.
Body and organ weight measurements
Initial and final body weights of the animals were recorded. At autopsy, the reproductive and accessory sex organs (testes, epididymis, seminal vesicle, ventral prostate and vas deferens) along with the liver were dissected out, freed from adherent tissues and weighed, slices of tissues were fixed in Bouin’s fluid, paraffin sections were made and stained with Ehrlich hematoxylin and eosin (CitationHumason, 1979).
Sperm motility and density
After anesthetizing the rat, the epididymis was exposed by scrotal incision, and spermatozoa were expressed out by cutting the distal end of the cauda epididymal tubule.
The motility of cauda epididymal spermatozoa was recorded and the percentage of motile sperms was calculated per unit area (CitationSrikanth et al., 1999). Cauda epididymal sperm density was assessed using a Neubauer’s hemocytometer (CitationZaneveld & Polakoski, 1977).
Assay of serum testosterone concentration
Serum concentration of testosterone was measured following an immunoenzymatic method with a ELISA reader (Merck, Japan), according to the standard protocol given by the National Institute of Health and Family Welfare (CitationShrivastava, 2002).
Hematology
Blood was collected and the values of red blood cell (RBC) and white blood cell (WBC) counts (CitationLynch et al., 1969), hematocrit (CitationStrumia et al., 1954), hemoglobin percentage (CitationCrossby et al., 1954), and blood sugar (CitationAstoor & King, 1954) were estimated.
Histological studies of testes
The testes of the animals were processed for histopathological evaluation. For histology the tissues were fixed in Bouin’s fluid, dehydrated, and embedded in paraffin for sections at 5 μm. These sections were stained in Harris hematoxylene and eosin.
Histometry
The evaluation of cell population dynamics was based on the calculations made for each cell type per cross section of seminiferous tubule. Various cell components were quantitatively analyzed using spherical tubules and a correcting factor was introduced (CitationBerndtson, 1977). Interstitial cell types such as fibroblast, mature and degenerating Leydig cells were estimated (CitationDixon & Massey, 1957).
Statistical analysis
All of the recorded values of body/organ weight, sperm dynamics, hematological parameters and testicular cell dynamics were expressed in terms of mean ± SEM. The treated groups were compared to control using the Student’s t-test (CitationIpstein & Poly, 1970).
Ethical aspects
The study was approved by the ethical committee of the University, Department of Zoology, Jaipur, India. Indian National Science Academy, New Delhi (CitationINSA, 2000) guidelines were followed for maintenance and use of the experimental animals.
Results
Weight response
The oral treatment of Myristica fragrans oil did not cause any significant change in the body weight of the treated rats with respect to the initial body weight (). However, the weight of testes, epididymis, seminal vesicle, ventral prostate and vas deferens decreased significantly (P < 0.001) with respect to the weights of the control group in all the treatment groups, while a non-significant change in the weight of liver was observed after the treatment. A non-significant change in the above-mentioned parameters was observed in Group V of the experiment after 120 days of recovery.
Table 1. Body and organs weight of Myristica fragrans oil treated male albino rats.
Sperm dynamics and fertility test
Results given in showed a significant reduction (P < 0.001) of sperm motility in cauda epididymis. The fertility test showed 55%, 85%, and 100% negative fertility in Group II, III, and IV, respectively. The number of implantation sites and the number of viable fetuses was recorded to be nil at the highest dose level tested. In Group V, sperm motility and sperm density showed non-significant changes and 80% positive fertility was observed after 120 days of recovery. In the recovery phase, the number of pregnant females was 16 out of the group of 20, and a non-significant change was observed in the values of the number of implantation sites and number of viable fetuses when compared with the normal group.
Table 2. Effect of Myristica fragrans oil on male rat fertility.
Serum testosterone levels
The concentration of serum testosterone was significantly decreased in all the treatment groups in a dose-dependent manner, while in the recovery group, the level of testosterone recovered to the control level ().
Table 3. Effect of Myristica fragrans oil on the level of serum testosterone.
Hematology
The hematological parameters (RBC – Red Blood Cell Count, WBC – White Blood Cell Counts, hemoglobin, hematocrit, MCV – Mean Corpuscular Volume, MCH – Mean Corpuscular Hemoglobin, MCHC – Mean Corpuscular Hemoglobin Concentration and blood sugar) remained unaffected in all the treatment and recovery groups ().
Table 4. Haematological changes of Myristica fragrans oil treated male rats.
Histology
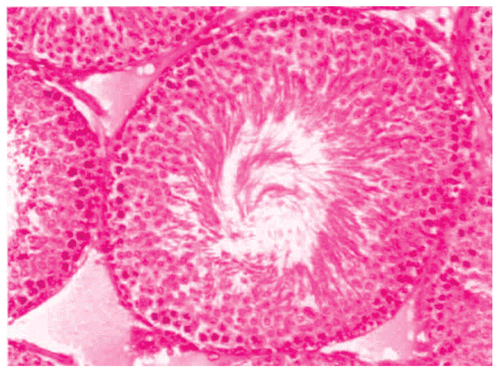
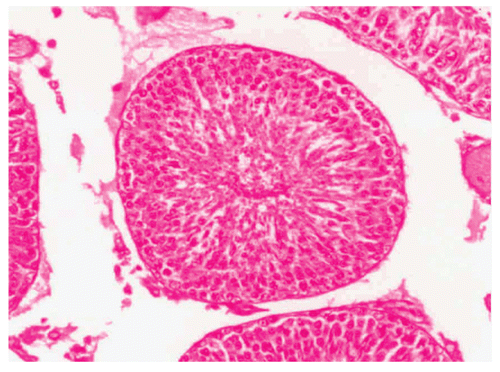
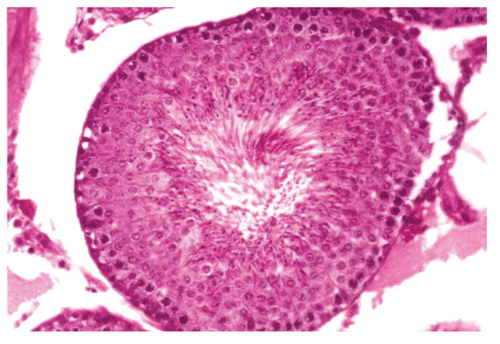
Histological studies of control rat testis show all successive stages of spermatogenesis, where the lumen was filled with sperm (). The sections of testis of Group II fed rats show partial damage of spermatogenic elements (). In Group III fed rats, the section of testis revealed a decrease in the diameter of seminiferous tubules and the lumen was devoid of spermatozoa while the Leydig cells were normal in appearance (). Completely degenerating spermatogenesis could be seen with the Myristica fragrans oil treatment group (), the main histological alterations observed were the decreased number of spermatogenic elements in the seminiferous tubule, cellular debris in lumen, increased interstitial space and damaged Leydig cells. The recovery group illustrated almost the control histoarchitecture of testis ().
Figure 3. Showing the section of Rat testis treated with Myristica fragrans Oil at the dose level of 0.2 ml/kg b. wt. for 60 days.
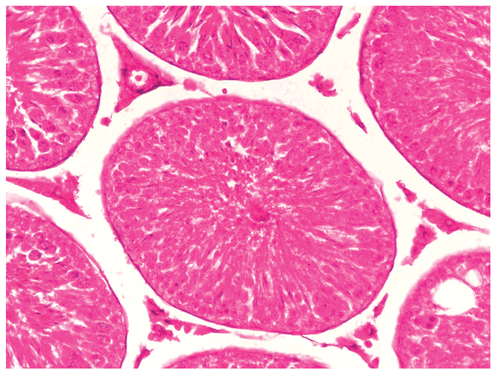
Figure 4. Showing the section of Rat testis treated with Myristica fragrans Oil at the dose level of 0.4 ml/kg b. wt. for 60 days.
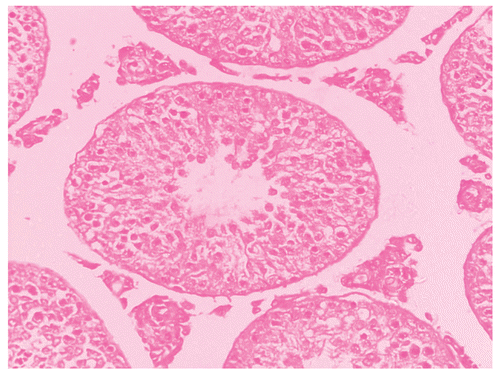
Histometry
Results presented in revealed that seminiferous tubule diameter and percentage of normal tubules decreased significantly (P <0.001) in all the treatment groups in a dose-dependent fashion, whereas the percentage of abnormal tubules increased significantly (P <0.001).
Figure 7. Effect of administration of Myristica fragrans Oil on the seminiferous tubule count of male albino rats.
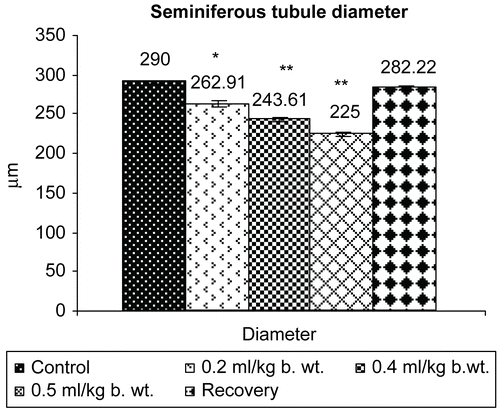
Figure 8. Effect of administration of Myristica fragrans Oil on the seminiferous tubule diameter of male albino rats.
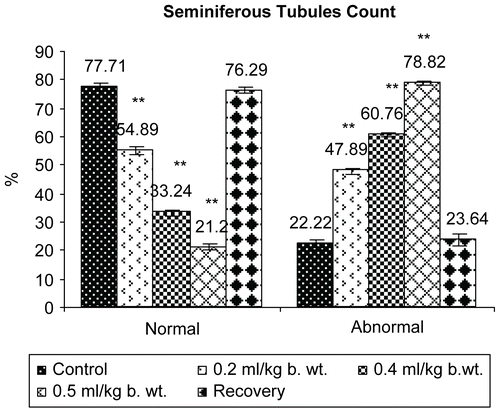
Figure 9. Effect of administration of Myristica fragrans Oil on the Leydig cell area of male albino rats.
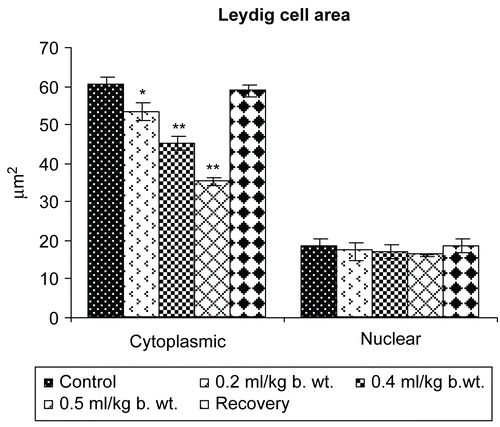
Figure 10. Effect of administration of Myristica fragrans Oil on the Leydig cell count of male albino rats.
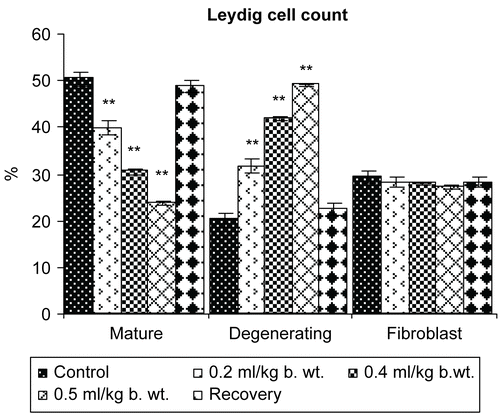
The cytoplasmic and nuclear area of Leydig cells showed a highly significant reduction (P <0.001). The percentage of differential count of mature Leydig cells was reduced significantly (P <0.01) and degenerating Leydig cells were increased significantly (P <0.001), whereas a non-significant decrease in the differential count of fibroblast like cells was recorded following the oral administration of Myristica fragrans oil ().
In Group V of 120 days of recovery, a non-significant change was recorded in the values of all parameters mentioned above when compared with the control animals.
Discussion
The oral treatment of male rats with Myristica fragrans oil showed a significant reduction in testis, epididymides, seminal vesicles, ventral prostate, and vas deferens weights. This may be due to low plasma level of testosterone, as testicular size is the best primary assessment of spermatogenesis, since the tubules and germinal elements account for approximately 88% of the testicular mass (CitationSherines et al., 1978). Reduction in the weight of accessory reproductive organs directly supports the reduced availability of androgens (CitationSharma & Jacob, 2001). The recovery phase showed complete recovery from all the effects mentioned above.
The reduction in sperm motility in cauda epididymis is of importance with regard to fertilization; it may be due to androgen deficiency (CitationChitra et al., 2001). The 100% negative fertility test may be attributed to decreased spermatozoa density and motility of cauda epididymis. Moreover, the spermatozoa were sluggishly motile and, therefore, unable to fertilize the normal cycling females. Reduction in the progressive epididymal sperm motility of the treated rats may be responsible for the severe decrease in the average number of implantation sites, viable fetuses and number of litters born by the female rats cohabited with Myristica fragrans oil-treated male rats leading to the presence of sperm-toxic agents in the oil (CitationVerma & Chinoy, 2001). The progressive mode in the recovery group suggested the deleterious effect was reversible.
A significant decline in serum testosterone might be due to the adverse effect of treatment on hormonal milieu of the testes. The reduced weights of seminal vesicle and ventral prostate further support the suppressed concentration of testosterone in circulation (CitationLohiya & Ansari, 1999).
No change in the values of hematological parameters in treated as well as in the recovery group showed that the drug did not affect the vital functioning of rats.
The seminiferous tubule diameters were decreased due to the absence of spermatogenic cells. The reduction in the number of spermatogenic cells may be due to the non-availability of testosterone, as spermatogenesis is activated by testosterone, which is synthesized by Leydig cells (CitationSharpe, 1987). The impairment of Leydig cell function was evident by its reduced nuclear area and lower number of mature Leydig cells. The number of mature Leydig cells had a direct effect on spermatogenesis. Deformation of Leydig cell and reduction in cauda epithelial cell height indicates the inefficiency of these cells to synthesize testosterone (CitationHulethal & Lunenfeld, 2004). The checking of treatment in the recovery group again normalized the process of spermatogenesis, thus maintaining the level of testosterone in the normal ranges.
These results suggested that oral administration of Myristica fragrans oil could lead to antifertile state in male rats due to the interference of the oil in the testicular androgen levels, which could recover after terminating the treatment.
Acknowledgements
The authors are thankful to the Centre for Advanced Studies, Department of Zoology, University of Rajasthan, Jaipur for providing necessary facilities.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Astoor AM, King EJ (1954): Simplified colorimetric blood sugar method. J Biochem 56: 44–55.
- Berndtson WE (1977): Methods for quantifying mammalian spermatogenesis: A review. J Am Sci 44: 818–833.
- Chitra KC, Latchaumycandane, Mathur PP (2001): Chronic effect of endosulfan on the testicular functions of rats. Asian J Androl 1: 203–206.
- Chopra RN, Nayar SL, Chopra IC (1956): Glossary of Indian Medicinal Plants. New Delhi, CSIR Publication.
- Crossby WH, Munn JI, Furth FW (1954): Standardizing a method for clinical haemoglobinometry. US Armed Force Med J 5: 695–703.
- Dixon W, Massey FJ (1957): Introduction to Statistical Analysis. New York, McGraw Hill, pp. 228.
- Grover JK, Khandkar S, Vats V, Dhunnoo Y, Das D (2002): Pharmacological studies on Myristica fragrans: Antidiarrheal, hypnotic, analgesic and hemodynamic (blood pressure) parameters. Methods Find Expt Clin Pharmacol 24: 675–680.
- Hulethal M, Lunenfeld E (2004): Regulation of spermatogenesis by paracrine/auto testicular factors. Asian J Androl 6: 259–260.
- Humason GL (1979): Animal Tissue Techniques. San Francisco, W.H. Freeman.
- INSA (2000): Guidelines for Care and Use of Animals in Scientific Research. New Delhi, Indian National Science Academy.
- Ipstein J, Poly F (1970): Brancroft’s Introduction to Biostatistics, 2nd edn. New York, Harper International, pp. 44.
- Lohiya NK, Ansari AS (1999): Male contraceptive agents. In: Joy CP, Krishna KP, Krishna A, Haldar C, eds, Comparative Endocrinology and Reproduction. New Delhi, Narosa Publishing House, pp. 260–277.
- Lynch JM, Raphel SS, Melier LD, Spare PD, Inwood MJM (1969): Collection of blood sample and haemocytometry - red cell count, white cell count. In: Medical Laboratory Technology and Clinical Pathology. Tokyo, W.B. Saunders, Igakar Sohm, pp. 626–662.
- Pecevski J, Savkovic D, Radivojevic D, Vuksanovic L (1981): Effect of oil of nutmeg on the fertility and induction of meiotic chromosome rearrangements in mice and their first generation. Toxicol Letter 7: 239–243.
- Sharma N, Jacob D (2001): Antifertility investigation and toxicological screening of the petroleum ether extract of the leaves of Mentha arvensis L. in male albino mice. J Ethanopharmacol 75: 5–12.
- Sharpe RM (1987): Testosterone and spermatogenesis. J Androl 113: 1–2.
- Sherines RJ, Howards SS (1978): Male Infertility in: Harrison JH, Gittes RF, Perimutter AD, Stamey TA and Walsh PC, eds, Campbell’s Urology, 4th edn. Philadelphia, Pa: W.B. Saunders, p. 715.
- Srivastava, TG (2002): Enzyme linked immuno sorbent assay for steroid hormones, in: Orientation Training Course on Research Methodology in Reproductive Biomedicine. New Delhi: National Institute of Health and Family Welfare, pp. 55–58.
- Sonavane GS, Sarveiya VP, Kasture VS, Kasture SB (2002): Anxiogenic activity of Myristica fragrans seeds. Pharmacol Biochem Behav 71: 239–244.
- Srikanth V, Malini T, Arunakaran J, Govindarajulu P, Balasubramanian K (1999): Effects of ethanol treatment on epididymal secretory products and sperm maturation in albino rats. J Pharmacol Expt Therapeutics 288: 509–515.
- Strumia MM, Sample AB, Hast ED (1954): An improved microhaematocrit method. American J Clinopathol 24: 1016–1024.
- Verma RJ, Chinoy NJ (2001): Effects of papaya seed extract on microenvironment of cauda epididymis. Asian J Androl 3: 143–146.
- WHO (1983): Protocol MB-50: a method for examining the effect of the plant extracts administered orally on the fertility of male rats. APF/IP, 9914E. Geneva, World Health Organization.
- Zaneveld LJD, Polakoski KL (1977): Collection and physical examination of the ejaculate, in: Hafez ESE, ed., Techniques of Human Andrology. Amsterdam: North Biomedical Press, pp. 147–156.