Abstract
In our continuous search of biological properties of Senecio species (Compositae), we investigated S. ambiguus subsp. ambiguus (Biv.) DC, S. gibbosus subsp. gibbosus DC, S. leucanthemifolius Poiret, S. inaequidens DC, and S. vulgaris L. for their angiotensin converting enzyme (ACE) inhibitory activity through an in vitro bioassay based on the enzymatic cleavage of the chromophore-fluorophore labelled substrate dansyltriglycine into dansylglycine, which is quantitatively measured by HPLC. Among analyzed extracts, ethyl acetate demonstrated the highest activity with IC50 values of 192.1 and 219.1 μg/mL for S. ambiguus subsp. ambiguus and S. inaequidens, respectively. Flavonoids were detected in these extracts on TLC sprayed with Natural Products reagent - polyethylene glycal reagent (NP/PEG).
Introduction
Hypertension is a common and often progressive disorder that poses a major risk for cardiovascular and renal disease (CitationChalmers, 1999; CitationOdama & Bakris, 2000). Recent data have revealed that the global burden of hypertension is an important and increasing public health problem worldwide and that the level of awareness, treatment, and control of hypertension varies considerably among countries (CitationKearney et al., 2005). From a pathophysiological point of view, it is important to note that hypertensive disease involves changes in at least one of three hemodynamic variables (cardiac output, arterial stiffness, or peripheral resistance) that determine the measurable blood pressure (CitationPerticone et al., 2001; CitationRizzoni et al., 2003). Each of these variables is a potential therapeutic target, and it is likely that changes in these variables also contribute to heterogeneity in the pharmacologic response of patients with hypertension.
Therefore, modern treatment strategies should not only target blood pressure reduction but also normalize vascular structure and function. Angiotensin converting enzyme (ACE) inhibitors are widely used in therapy, demonstrating their efficacy in reducing blood pressure, reversing abnormalities of vascular structure and function in patients with essential hypertension, and ultimately preventing “global cardiovascular risk” (CitationBrown & Hall, 2005). ACE is a cell membrane peptidase, working as an ecto-enzyme, with its catalytic site exposed at the extracellular surface of the cell; it catalyzes conversion of angiotensin I into the active angiotensin II. This octapeptide is directly or indirectly involved in bradykinin metabolism. Inhibition of ACE is an effective screening method in the search of new antihypertensive agents (Wagner, 1991; CitationHansen et al., 1995; CitationSomanadhan et al., 1996).
For a long time medicinal plants have been used for the treatment of many diseases, in most cases without a scientific background supporting their use. At present there is increasing emphasis on determining the scientific evidence and rationale for the use of preparation from medicinal plants.
Senecio is the largest and complex genus in the tribe Senecionae (Compositae) and more than 1300 species have been reported (CitationLoyola et al., 1985). This genus is rich in pyrrolizidine alkaloids (PAs) and sesquiterpenes with a furanoeremophilane skeleton while just few studies report about components like chalcones or flavonoids (CitationBohlmann et al., 1986; CitationUrones et al., 1988; CitationPérez et al., 1991).
Senecio species have been used in folk medicine in the treatment of wounds and as antiemetic, anti-inflammatory and vasodilator preparations (CitationRose, 1972; CitationBautista Perez et al., 1991; CitationPérez et al., 1999). In a previous study, dihydroeuparin, a compound with strong antihypertensive activity was isolated from S. graveolens used as tea infusion for the treatment of mountain sickness (CitationLoyola et al., 1985).
In our continuing screening on the antihypertensive activity of Senecio species (CitationTundis et al., 2005), we report for the first time the ACE inhibitory activity of S. ambiguus subsp. ambiguus (Biv.) DC, S. gibbosus subsp. gibbosus DC, S. leucanthemifolius Poiret, S. inaequidens DC, and S. vulgaris L. extracts.
Materials and methods
Plant material
The aerial part of Senecio ambiguus subsp. ambiguus (Biv.) DC, Senecio gibbosus subsp. gibbosus DC, Senecio leucanthemifolius Poiret, Senecio inaequidens DC, and Senecio vulgaris L. were collected in southern Italy during flowering season 2003 in their natural habitat. Plant materials were taxonomically identified by N.G. Passalacqua and L. Peruzzi of the Natural History Museum of Calabria and Botanical Garden of University of Calabria, Italy. Voucher specimens were deposited in the Botany Department Herbarium at the University of Calabria (CLU), Italy. All species were dried in a dark place at room temperature and coarsely powdered before extraction.
Extraction procedure
Dried and powdered aerial parts of S. ambiguus subsp. ambiguus (Biv.) DC, S. gibbosus subsp. gibbosus DC, S. leucanthemifolius Poiret, S. inaequidens DC, and S. vulgaris L. (200 g) were extracted exhaustively with methanol (3 × 5 L) at room temperature to give the crude extract. Crude extract was suspended in H2O and partioned with n-hexane, dichloromethane (CH2Cl2) and ethyl acetate (EtOAc). The residue was acidified with 2.5% H2SO4 and stirred overnight with Zn powder to reduce pyrrolizidine alkaloids (PAs) N-oxides. The aqueous acid solution was basified, and extracted with chloroform (CHCl3). The combined organic solutions were dried over anhydrous sodium sulphate and evaporated to dryness. Yield of extracts are reported in .
Table 1. Content (g) of methanol extracts and fractions of Senecio species.
Composition of extracts
In order to identify the inhibitory compounds present within the Senecio extracts silica gel Thin Layer Chromatography (TLC) was carried out and specific spray reagents used for various compound types (Wagner & Bladt, 1996).
The n-hexane extracts were developed with n-hexane:acetone (6:4) and then treated with vanillin-sulphuric acid reagent (VS) highlighting the presence of terpenoids. The EtOAc extracts were developed with EtOAc:HCOOH:AcOH:H2O (100:10:10:20) and then sprayed with Natural Products reagent-polyethylene glycol reagent (NP/PEG) revealing the presence of flavonoids when examined under UV light 365 nm in all Senecio species except in S. leucanthemifolius. Alkaloids were detected in chloroform extracts eluting with CH2Cl2-MeOH-NH3 85:14:1, immediately followed by spraying with Dragendorff’s reagent (DRG).
ACE-inhibition test
The in vitro ACE inhibitory activity was measured using the method described by CitationElbl and Wagner (1991), which was later modified by CitationHansen et al. (1995). Briefly, the chromophore-fluorophore labeled substrate dansyltriglycine was cleaved by angiotensin I-converting enzyme preparation from rabbit lung (EC 3.4.15.1) into dansylglycine, which is quantitatively measured by HPLC.
The test extract (1 mg) was dissolved in 1 mL HEPES assay buffer, to obtain the final concentration from 330 to of 100 μg/mL of inhibitors solution. The ACE solution (25 μL) was pre-incubated in a test or control solution (25 μL) for 5 min at 37°C. The enzyme reaction was started by adding a combined solution (25 μL) of the substrate dansyltriglycine (7.86 mM), and the internal standard, dansyl-l-glutamine (0.353 mM). At the end of the incubation time the reaction was stopped by adding a solution of 0.1 N Na2EDTA (50 μL). The dansylglycine and dansyltriglycine were separated and quantified by reversed phase HPLC with UV detection at 250 nm.
Instrumentation
HPLC Perkin Elmer Series 410 LC pump; Injector Perkin Elmer 20 μL loop. Detector Perkin Elmer UV/VIS LC290 spectophotometric; solvent system: ALTECH SN 1250-99, Part No. 288215 BIN II 43, HYPERSIL ODS 5u Lot No. 5002. 150 mm × 4.6 mm SN:1250-99; mobile phase: isocratic system- 10 mM NaH2PO4 buffer (pH 7):acetonitrile (88:12); flow rate 2 mL/min, run time 30 min. Linear calibration curve for dansylglycine was plotting from 0.2 to 25 μg/mL. All materials were purchased from Sigma-Aldrich, Milan, Italy.
Tannin test
The extracts inhibiting ACE by 50% or more were subjected to the gelatin salt block test to eliminate false positives brought about by the presence of tannins. The tannins test was performed by extracting 5 g of dried plant materials with 50 mL of water, ethanol (96%) or acetone. After evaporation of the solvents, the extracts were re-dissolved in 13 mL hot water (90-100°C) and allowed to cool to room temperature. Two drops of 10% NaCl are added to “salt” out any non-tannin compounds which could cause a false positive reaction. After vacuum filtration, 3 mL of filtrate was added to each of four test tubes. The following solutions were then added to the test tubes: 4-5 drops of 1% gelatin solution; 4-5 drops of 1% gelatin + 10% NaCl solution; and 3-4 drops of 10% ferric chloride. For a negative control water was used without extract. The test was considered negative if there was no precipitation in tubes 1 and 2 or if 3 showed no color formation, and positive if there was precipitation in tubes 1 and 2 and color formation in 3 (either blue-black for hydrolysable or brownish-green for condensed tannins) (CitationNyman et al., 1998).
Results and discussion
Pyrrolizidine alkaloids (PAs) are the most characteristic secondary metabolites of Senecio species (CitationHartmann & Ober, 2000). PAs are ester alkaloids consisting of a necine base moiety, esterified with a necic acid. They may occur as monoesters, open-chain diesters, or macrocyclic diesters. In all Senecio species, senecionine N-oxide was identified as the primary product of biosynthesis. It is synthesized in the roots and translocated into the shoots, where it is transformed into the species-specific PA profiles (CitationPelser et al., 2005). Senecionine was proved to be incorporated into simple retronecine esters as seneciphylline; epoxides of retronecine esters as jacobine including jaconine, its product of chlorolysis, jacozine; and epoxides of otonecine esters as otosenine and florosenine.
Although pyrrolizidine alkaloids (PAs) are known for their hepatotoxicity, mutagenicity carcinogenicity, and teratogenicity (CitationFu et al., 2004), several biological activities including ACE inhibitory activity of some Senecio species have been reported (CitationEl-Shazly et al., 2002; CitationToma et al., 2004; CitationLoizzo et al., 2004, Citation2005, Citation2006, Citation2007; CitationTundis et al., 2005; CitationConforti et al., 2006). For the above mentioned reason, PAs were removed from methanol extracts using chloroform following the procedure previously described. These extracts were not tested for ACE inhibition.
ACE inhibition was revealed through an in vitro bioassay based on the measure of the enzymatic cleavage of the chromophore-fluorophore-labeled substrate dansyltriglycine into dansylglycine and diglycine. The decreased concentration of dansylglycine in the test reaction compared with the control reaction was expressed as percentage inhibition and calculated from the equation:
where T = test reaction and C = control reaction.
The IC50 values of S. ambiguus subsp. ambiguus, S. gibbosus subsp. gibbosus, S. leucanthemifolius, S. inaequidens, S. vulgaris extracts are summarized in . Among the tested extracts, it is interesting to note that dicloromethane extracts did not exhibit any type of activity according to S. samnitum biological profile (CitationTundis et al., 2005). Among analysed n-hexane extracts, S. ambiguus subsp. ambiguus demonstrated considerable activity with an IC50 of 306.9 μg/mL. This ACE inhibitory property may be due to the presence of compounds such as linoleic acid, palmitic acid, γ-tocopherol, α- and β-amyrin, and campesterol, identified by GC/MS analysis in this species in our previous work (Tundis et al., Citation2005a). Differentially, all EtOAc extracts exhibited significant activity and dose-response curve was shown in in this work. In particular, S. inaequidens exhibited the highest ACE inhibitory with an IC50 value of 192.1 μg/mL. S. ambiguus subsp. ambiguus showed a strong activity with an IC50 of 219.1 μg/mL.
Figure 1. Dose-dependent inhibition of ACE by Senecio species EtOAc and n-hexane extracts. Each data point represents the mean ± SD (n = 3).
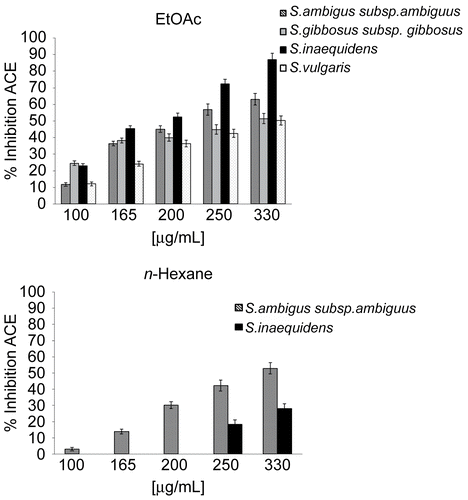
Table 2. ACE inhibitory activity (IC50) of Senecio species (μg/mL).
The same bioactive profile was observed for S. gibbosus subsp. gibbosus and S. vulgaris, exhibiting ACE inhibition activity in only EtOAc extracts, with IC50 values of 309.4 and 327.8 μg/mL, respectively. CitationFerry (1977) isolated and identified quercetin, isoquercetrin, isorhamnetin-3-O-rutinoside, quercetin-3-O-glucoside and some polyphenols such as phenolic acid, vanillic acid, and caffeic acid in S. inaequidens and S. vulgaris. We believe that flavonoids are responsible for the observed ACE inhibitory activity; in fact, CitationWagner et al. (1991) demonstrated how flavonoids can inhibit ACE through the generation of chelate complexes within the active center of ACE. Free hydroxyl groups of phenolic compounds are also suggested to be important structural moieties to chelate the zinc ions, thus inactivate the ACE activity (CitationChen & Lin, 1992). The non-flavonoid polyphenol compounds failed to inhibit ACE activity. This lack of effect on ACE activity does not allow the establishment of any structure-activity relationship, like that observed for membrane interaction and antioxidant capacity (CitationVerstraeten et al., 2003; CitationErlejman et al., 2004).
On the contrary, S. leucanthemifolius EtOAc extract was inactive against ACE, possibly due to the lack of flavonoids in this species compared to the others analyzed in this work. A tannin test was done on EtOAc extracts in order to eliminate false positive. Moreover, in our previous investigation we reported the S. inaequidens EtOAc extract possesses greater antioxidant activity in comparison with S. vulgaris and this different biological profile was linked to the different flavonoid content (Conforti et al., Citation2006a).
The present work showed for the first time the antihypertensive properties of different Senecio species and scientifically supports the traditional use of Senecio. Among analyzed extracts, EtOAc exhibited the most promising activity, probably due to the presence of flavonoids. Further research relating to isolation of the active constituents is in progress in our laboratory.
Acknowledgements
The authors wish to thank Dr. N.G. Passalacqua and Dr. L. Peruzzi of the Botany Department of University of Calabria, Italy for the identification and collection of the plant materials, and Dr. V. Filippelli for the English revision of this manuscript.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bautista Peresz J, Stubing G, Figuerola R (1991): Guia de las Plantas Medicinales de la Communidad Valenciana. Valencia, Las Provincias, pp. 27–29.
- Bohlmann F, Zdero C, Jakupovic J, Grenz M, Castro V, King RM, Robinson H, Vincent LPD (1986): Further pyrrolizidine alkaloids and furoeremophilanes from Senecio species. Phytochemistry 25: 1151–1159.
- Brown B, Hall AS (2005): Renin-angiotensin system modulation: the weight of evidence. Am J Hypertens 18: 127S–133S.
- Chalmers J (1999): World Health Organization–International Society of Hypertension guidelines for the management of hypertension. Blood Press 8: 9–43.
- Chen CH, Lin JY (1992): Inhibition of angiotensin-I-converting enzyme by tetrahydroxyxanthones isolated from Tripterospermum lanceolatum. J Nat Prod 55: 691–695.
- Conforti F, Marrelli M, Statti G, Menichini F (2006): Antioxidant and cytotoxic activities of methanolic extract and fractions from Senecio gibbosus subsp. gibbosus (GUSS) DC. Nat Prod Res 20: 805–812.
- Conforti F, Loizzo MR, Statti GA, Houghton PJ, Menichini F (2006a): Biological properties of different extracts of two Senecio species. Int J Food Sci Nutr 57: 1–8.
- Elbl G, Wagner H (1991): A new method for the in vitro screening of inhibitors of angiotensin converting enzyme (ACE), using the cromophore- and fluorophore-labelled substrate, dansyltriglicine. Planta Med 57: 137–141.
- El-Shazly A, Dorai G, Wink M (2002): Chemical composition and biological activity of the essential oils of Senecio aegyptius var. discoideus Boiss. Z Naturforsch 57c: 434–439.
- Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI (2004): The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects. Free Radic Res 38: 1311–1320.
- Ferry S (1977): Sur les polyphénols (acides-phénols, flavonoïd) de quelques Séneçons indigènes. Plantes Méd Phytothérapie XI: 25–39.
- Fu PP, Xia Q, Lin G, Chou MW (2004): Pyrrolizidine alkaloids genotoxicity, metabolism enzymes, metabolic activation, and mechanisms. Drug Metab Rev 36: 1–55.
- Hansen K, Nyman U, Wagner Smith U, Adersen A, Gudiksen L, Rajasekharan S, Pushpangadan P (1995): In vitro screening of traditional medicines for anti-hypertensive effect based on inhibition of the angiotensin converting enzyme (ACE). J Ethnopharmacol 48: 43–51.
- Hartmann T, Ober D (2000): Chemistry, Biology, and Chemoecology of the Pyrrolizidine Alkaloids. in: Leeper LJ, Vederas JC (Eds.). Topics in Current Chemistry 209, Heidelberg, Springer-Verlag, pp. 207–247.
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J (2005): Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223.
- Loizzo MR, Statti GA, Tundis R, Conforti F, Bonesi M, Autelitano G, Houghton PJ, Miljkovic-Brake A, Menichini F (2004): Antibacterial and antifungal activity of Senecio inaequidens DC. and Senecio vulgaris L. Phytother Res 18: 777–779.
- Loizzo MR, Tundis R, Statti GA, Menichini F, Houghton PJ (2005): In vitro antiproliferative effects on human tumor cell lines of extracts and jacaranone from Senecio leucanthemifolius Poiret. J Pharm Pharmacol 57: 897–902.
- Loizzo MR, Tundis R, Statti GA, Miljkovic-Brake A, Menichini F, Houghton, PJ (2006): Bioactive extracts from Senecio samnitum Huet. Nat Prod Res 20: 265–269.
- Loizzo MR, Tundis R, Statti GA, Menichini F (2007): Jacaranone: A cytotoxic constituent from Senecio ambiguus subsp. ambiguus (Biv.) DC. against renal adenocarcinoma ACHN and prostate carcinoma LNCaP cells. Arch Pharm Res 30: 700–707.
- Loyola LA, Pedreros S, Morales G (1985): p-Hydroxyacetophenone derivatives from Senecio graveolens. Phytochemistry 24: 1600–1602.
- Nyman U, Joshi P, Madsen LB, Pedersen TB, Pinstrup M, Rajasekharan S, George V, Pushpangadan P (1998): Ethnomedical information and in vitro screening for angiotensin-converting enzyme inhibition of plants utilized as traditional medicines in Gujarat, Rajasthan and Kerala (India). J Ethnopharmacol 60: 247–263.
- Odama U, Bakris GL (2000): Target organ damage in hypertension. J Clin Hypertens 2: 312–318.
- Pelser BP, de Vos H, Theuring C, Beuerle T, Vrieling K, Hartmann T (2005): Frequent gain and loss of pyrrolizidine alkaloids in the evolution of Senecio section Jacobaea (Asteraceae). Phytochemistry 66: 1285–1295.
- Pérez AL, Vidales P, Cardenas J, De Vivar R (1991): Eremophilanolides from Senecio toluccanus var. modestus. Phytochemistry 30: 905–908.
- Pérez C, Agnese AM, Cabrera JL (1999): The essential oil of Senecio graveolens (Compositae): chemical composition and antimicrobial activity tests. J Ethnopharmacol 66: 91–96.
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G (2001): Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 104: 191–196.
- Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E (2003): Prognostic significance of small-artery structure in hypertension. Circulation 108: 2230 -2235.
- Rose EF (1972): Senecio species: Toxic plants used as food and medicine in the Transkei. S Afr Med 46: 1039–1043.
- Somanadhan B, Varughese G, Palpu P, Sreedharan R, Gudiken L, Wagner Smitt U, Nyman U (1996): An ethnopharmacological survey for potential angiotensin converting enzyme inhibitors from Indian medicinal plants. J Ethnopharmacol 65: 103–112.
- Toma W, Trigo JR, Bensuaski de Paula AC, Monteiro Souza Brito AR (2004): Preventive activity of pyrrolizidine alkaloids from Senecio brasiliensis (Asteraceae) on gastric and duodenal induced ulcer on mice and rats. J Ethnopharmacol 95: 345–351.
- Tundis R, Loizzo MR, Statti GA, Deguin B, Amissah R, Houghton PJ, Menichini F (2005): Chemical composition and angiotensin-converting enzyme activity Senecio samnitum Huet. Pharm Biol 43: 605–608.
- Tundis R, Passalacqua NG, Peruzzi L, Statti GA, Bonesi M, Loizzo MR, Conforti F, Cesca G, Menichini F (2005a): Comparative chemical variability of the non-polar extracts from Senecio cineraria group (Asteraceae). Biochem Syst Ecol 33: 1071–1076.
- Urones JG, Barcala PB, Marcos IS, Moro RF, Esteban Lòpez M, Rodriguez F (1988): Pyrrolizidine alkaloids from Senecio gallicus and Senecio adonifolius. Phytochemistry 27: 1507–1510.
- Verstraeten SV, Keen CL, Schmitz HH, Fraga CG, Oteiza PI (2003): Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic Biol Med 1: 84–92.
- Wagner H, Elbl G, Lotter H, Uinea M (1991): Evaluation of natural products as inhibitors of angiotensin I-converting enzyme (ACE). Pharm Pharmacol Lett 1: 15–18.
- Wagner H, Bladt S. (1996): Plant Drug Analysis, Springer, Berlin, pp. 359–360.
